Abstract
Purpose
To develop spherulite formulations to achieve high entrapment efficiency for both small and macromolecules as well as cell-type specific delivery.
Methods
Spherulites of various compositions were prepared, and lipid-PEG was incorporated through post-insertion. Calcein and FITC-labeled albumin were employed as model drugs for small and macromolecules. The spherulites were characterized with respect to entrapment efficiency, size, structure, and release kinetics, and the morphology was examined via cryo-EM. Finally, SV119-decorated spherulites were examined for their selective uptake by cancer cells.
Results
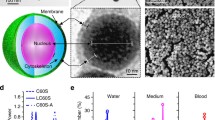
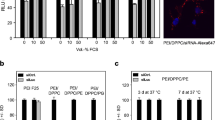
The spherulites are 170 ~ 290 nm in size. A loading efficiency of 55 ~ 60% can be consistently achieved for both calcein and albumin under optimized conditions. Cryo-EM shows the onion-like morphology consistent with the structure of multilamellar liposomes. A t1/2 of 39.3 h and 69.7 h in cargo release in serum was observed before and after PEG decoration, and incorporation of SV119 led to selective delivery of rhodamine-labeled spherulites to PC-3 tumor cells.
Conclusions
Our optimized formulations may represent a platform with simple preparation approach, relatively small particle size, high drug loading efficiency for both low and high molecular weight agents, and slow release kinetics for selective delivery of various types of therapeutics to target cells.







Similar content being viewed by others
Abbreviations
- BS10:
-
brij S10
- BSA:
-
bovine serum albumin
- CH:
-
cholesterol
- Cryo-EM:
-
cryo-electron microscopy
- Dex:
-
dexamethasone
- DLS:
-
dynamic light scattering
- DMEM:
-
Dulbecco’s modified eagle’s medium
- DPBS:
-
Dulbecco’s phosphate buffered saline
- DSPE-PEG2k :
-
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000]
- EE:
-
entrapment efficiency
- EPR:
-
enhanced permeability and retention
- FBS:
-
fetal bovine serum
- FITC:
-
fluorescein isothiocyanate
- FITC-BSA-SU:
-
fluorescein isothiocyanate-labeled succinylated bovine serum album
- MeO-PEG5k :
-
methoxypoly(ethylene glycol)-5000
- MLV:
-
multilamellar vesicles
- SPC:
-
soybean L-α-phosphatidylcholine
- TW80:
-
polyoxyethylene 80 sorbitan monooleate
References
Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–55.
Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318.
Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16.
Sutton D, Nasongkla N, Blanco E, Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24(6):1029–46.
Forrest ML, Yáñez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM. Paclitaxel prodrugs with sustained release and high solubility in poly(ethylene glycol)-b-poly(e-caprolactone) micelle nanocarriers: pharmacokinetic disposition, tolerability, and cytotoxicity. Pharm Res. 2008;25(1):194–206.
Fonseca MJ, Jagtenberg JC, Haisma HJ, Storm G. Liposome-mediated targeting of enzymes to cancer cells for site-specific activation of prodrugs: comparison with the corresponding antibody–enzyme conjugate. Pharm Res. 2003;20(3):423–8.
Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8(5):660–8.
Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4(4):297–305.
Abu Lila AS, Ishida T, Kiwada H. Targeting anticancer drugs to tumor vasculature using cationic liposomes. Pharm Res. 2010;27(7):1171–83.
Lian T, Ho RJY. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90(6):667–80.
Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–5.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–84.
Xiang G, Wu J, Lu Y, Liu Z, Lee RJ. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. Int J Pharm. 2008;356(1–2):29–36.
Gantert M, Lewrick F, Adrian JE, Rössler J, Steenpaß T, Schubert R, et al. Receptor-specific targeting with liposomes in vitro based on sterol-PEG1300 anchors. Pharm Res. 2009;26(3):529–38.
Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151(2):201–15.
Diat O, Roux D, Nallet F. Effect of shear on a lyotropic lamellar phase. J Phys II France. 1993;3(9):1427–52.
Diat O, Roux D. Preparation of monodisperse multilayer vesicles of controlled size and high encapsulation ratio. J Phys II France. 1993;3(1):9–14.
Redkar M, Hassan PA, Aswal V, Devarajan P. Onion phases of PEG-8 distearate. J Pharm Sci. 2007;96(9):2436–45.
Gauffre F, Roux D. Studying a new type of surfactant aggregate (“spherulites”) as chemical microreactors. A first example: copper ion entrapping and particle synthesis. Langmuir. 1999;15(11):3738–47.
Mignet N, Brun A, Degert C, Delord B, Roux D, Helene C, et al. The spherulitesTM: a promising carrier for oligonucleotide delivery. Nucleic Acids Res. 2000;28(16):3134–42.
Zhang Y, Huang Y, Zhang P, Gao X, Gibbs RB, Li S. Incorporation of a selective σ-2 receptor ligand enhances uptake of liposomes by multiple cancer cells. Int J Nanomedicine. 2012;7:4473–85.
Zhang P, Ye H, Min T, Zhang C. Water soluble poly(ethylene glycol) prodrug of silybin: design, synthesis, and characterization. J Appl Polym Sci. 2008;107(5):3230–5.
Uster PS, Allen TM, Danie BE, Mendez CJ, Newman MS, Zhu GZ. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 1996;386(2–3):243–6.
Szoka F. Comparative properties and methods of preparation of lipid vesicles (liposomes). Ann Rev Biophys Bioeng. 1980;9:467–508.
Simard P, Hoarau D, Khalid MN, Roux E, Leroux J-C. Preparation and in vivo evaluation of PEGylated spherulite formulations. Biochim Biophys Acta. 2005;1715(1):37–48.
Sułkowski WW, Pentak D, Nowak K, Sułkowska A. The influence of temperature, cholesterol content and pH on liposome stability. J Mol Struct. 2005;744:737–47.
Demel RA, Kinsky SC, Kinsky CB, van Deesen LLM. Effects of temperature and cholesterol on the glucose permeability of liposomes prepared with neutral and synthetic lecithins. Biochim Biophys Acta. 1968;150(4):655–65.
Demel RA, De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976;457(2):109–32.
Kim JC, Kim JD. Release property of temperature-sensitive liposome containing poly(N-isopropylacrylamide). Colloids Surf B. 2002;24(1):45–52.
Mu X, Zhong Z. Preparation and properties of poly(vinyl alcohol)-stabilized liposomes. Int J Pharm. 2006;318(1–2):55–61.
Senior JH. Fate and behaviour of liposomes in vivo: a review of controlling factors. Crit Rev Ther Drug Carrier Syst. 1987;3(2):123–93.
Semple SC, Chonn A, Cullis PR. Interactions of liposomes and lipid-based carrier systems with blood proteins: relation to clearance behaviour in vivo. Adv Drug Deliv Rev. 1998;32(1–2):3–17.
Nikolova AN, Jones MN. Effect of grafted PEG-2000 on the size and permeability of vesicles. Biochim Biophys Acta. 1996;1304(2):120–8.
Chonn A, Cullis PR. Ganglioside GM1 and hydrophilic polymers increase liposome circulation times by inhibiting the association of blood proteins. J Liposome Res. 1992;2(3):397–410.
Blume G, Cevc G. Liposomes for the sustained drug release in vivo. Biochim Biophys Acta. 1990;1029(1):91–7.
Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor-cell lines. Cancer Res. 1995;55(2):408–13.
Wheeler KT, Wang LM, Wallen CA, Childers SR, Cline JM, Keng PC, et al. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br J Cancer. 2000;82(6):1223–32.
Mach RH, Smith CR, AlNabulsi I, Whirrett BR, Childers SR, Wheeler KT. Sigma-2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997;57(1):156–61.
Colabufo NA, Berardi F, Contino M, Ferorelli S, Niso M, Perrone R, et al. Correlation between sigma2 receptor protein expression and histopathologic grade in human bladder cancer. Cancer Lett. 2006;237(1):83–8.
Kashiwagi H, McDunn JE, Simon PO, Goedegebuure PS, Xu J, Jones L, et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Mol Cancer. 2007;6(48):48–59.
Spitzer D, Simon Jr PO, Kashiwagi H, Xu J, Zeng C, Vangveravong S, et al. Use of multifunctional sigma-2 receptor ligand conjugates to trigger cancer-selective cell death signaling. Cancer Res. 2012;72(1):201–9.
Wang Y, Xu J, Xia X, Yang M, Vangveravong S, Chen J, et al. SV119-gold nanocage conjugates: a new platform for targeting cancer cells via sigma-2 receptors. Nanoscale. 2012;4(2):421–4.
Acknowledgments and Disclosures
This work was supported in part by NIH grants R01HL091828, R21CA128415 and R21CA155983.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, P., Huang, Y., Makhov, A.M. et al. Characterization of Spherulites as a Lipidic Carrier for Low and High Molecular Weight Agents. Pharm Res 30, 1525–1535 (2013). https://doi.org/10.1007/s11095-013-0990-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-0990-y