Abstract
Purpose
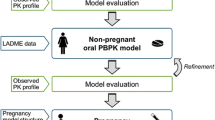
Physiological changes during pregnancy can effect pharmacokinetic (PK) parameters, which may lead to altered dose requirements. We aimed to leverage literature-based physiological changes during pregnancy into a PK model and compare its performance to a published reference model in pregnant women and to use the literature-based model to determine informative PK sampling times for a clinical study that aims to quantify the PK of enoxaparin throughout pregnancy.
Methods
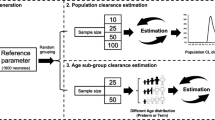
Changes in total body water (BW) and creatinine clearance (CRCL) during pregnancy were described using regression models. BW and CRCL were linked to a PK model of enoxaparin in non-pregnant women. Performance of the literature-based PK model was compared to a previously published empirical reference model. D-optimal sampling times were determined and evaluated for literature-based and reference models.
Results
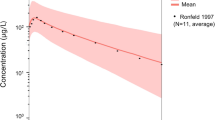
The literature-based model adequately predicted anti-Xa plasma concentrations when compared to reference model predictions. An informative sampling design was succesfully developed, with parameters expected with good precision (RSE < 36.4%).
Conclusion
A literature-based model describing enoxaparin PK during pregnancy was developed and evaluated. The modelling framework could be used to support development of informative designs in pregnancy when prior models are unavailable.



Similar content being viewed by others
Abbreviations
- BW:
-
body water
- BW0 :
-
pre-pregnancy body water
- BWMAX :
-
maximum change in body water
- BW50 :
-
half-maximum change in body water
- BWγ :
-
Hill coefficient for body water
- CL:
-
clearance
- CLNP :
-
non-pregnant clearance
- CLNR :
-
non-renal clearance
- CLR :
-
renal clearance
- CRCL:
-
creatinine clearance
- CRCL0 :
-
non-pregnant creatinine clearnace
- CRCLMAX :
-
maximum change in creatinine clarance
- CRCL50 :
-
half-maximum change in creatinine clearance
- f e :
-
fraction of renal clearance
- IU:
-
international units
- PD:
-
pharmacodynamics
- PK:
-
pharmacokinetics
- RSE:
-
relative standard error
- RUV:
-
residual unexplained variability
- SCR:
-
serum creatinine
- V:
-
volume
- VNP :
-
non-pregnant volume of distribution
- WT:
-
body weight
- WT0:
-
pre-pregnancy body weight
References
Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989.
Davison JM, Noble MCB. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88:10–7.
Dawes M, Chowienczyk PK. Pharmacokinetics in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2001;15:819–26.
Duffull S, Waterhouse T, Eccleston J. Some considerations on the design of population pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2005;32:441–57.
Andrew MA, et al. Amoxicillin pharmacokinetics in pregnant women: modeling and simulations of dosage strategies. Clin Pharmacol Ther. 2007;81:547–56.
Lebaudy, C. et al. Changes in enoxaparin pharmacokinetics during pregnancy and implications for antithrombotic therapeutic strategy. Clinical Pharmacology & Therapeutics (2008).
Andrew MA, Hebert MF, Vicini P. Physiologically based pharmacokinetic model of midazolam disposition during pregnancy. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2008;2008:5454–7.
Bergmann J-F, Mouly S. Thromboprophylaxis in medical patients: focus on France. Semin Thromb Hemost. 2002;28 Suppl 3:51–5.
Wong GC. Use of low-molecular-weight heparins in the management of acute coronary artery syndromes and percutaneous coronary intervention. JAMA. 2003;289:331–42.
Macklon NS, Greer I. Venous thromboembolic disease in obstetrics and gynaecology: the Scottish experience. Scott Med J. 1996;41:83–6.
Pabinger I, Schneider B. Thrombotic risk in hereditary antithrombin III, protein C, or protein S deficiency. A cooperative, retrospective study. Gesellschaft fur Thrombose- und Hamostaseforschung (GTH) Study Group on Natural Inhibitors. Arterioscler Thromb Vasc Biol. 1996;16:742–8.
Friederich PW, et al. Frequency of pregnancy-related venous thromboembolism in anticoagulant factor-deficient women: implications for prophylaxis. Ann Intern Med. 1996;125:955–60.
Conard J, Horellou M, Van Dreden P, Lecompte T, Samama M. Thrombosis and pregnancy in congenital deficiencies in AT III, protein C or protein S: study of 78 women. Thromb Haemost. 1990;63:319–20.
Green B, et al. Dosing strategy for enoxaparin in patients with renal impairment presenting with acute coronary syndromes. Br J Clin Pharmacol. 2005;59:281–90.
Toglia MR, Weg JG. Venous thromboembolism during pregnancy. N Engl J Med. 1996;335:108–14.
Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26:24–38.
Ensom MHH, Stephenson MD. Pharmacokinetics of low molecular weight heparin and unfractionated heparin in pregnancy. J Soc Gynecol Investig. 2004;11:377–83.
Greer I, Hunt BJ. Low molecular weight heparin in pregnancy: current issues. Br J Haematol. 2005;128:593–601.
Casele HL, Laifer SA, Woelkers DA, Venkataramanan R. Changes in the pharmacokinetics of the low-molecular-weight heparin enoxaparin sodium during pregnancy. Am J Obstet Gynecol. 1999;181:1113.
Duffus GM, MacGillivray I, Dennis KJ. The relationship between baby weight and changes in maternal weight, total body water, plasma volume, electrolytes and proteins and urinary oestriol excretion. Int J Obstet Gynaecol. 1971;78:97–104.
Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr. 1988;47:942.
Hytten FE, Thomson AM, Taggart N. Total body water in normal pregnancy. J Obstet Gynaecol Br Commonwealth. 1966;73:553–61.
Pipe NGJ, et al. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol. 1979;86:929–40.
Davison JM, Hytten FE. Glomerular filtration during and after pregnancy. J Obstet Gynaecol Br Commonwealth. 1974;81:588–95.
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science (New York, NY). 1997;276:122–6.
Holford NHG. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30:329–32.
Ochsenbein-Kolbe N, Roos M, Gasser T, Zimmermann R. Cross-sectional study of weight gain and increase in BMI throughout pregnancy. Eur J Obstet Gynecol. 2007;130:180–6.
Duffull SB, Dooley MJ, Green B, Poole SG, Kirkpatrick CMJ. A standard weight descriptor for dose adjustment in the obese patient. Clin Pharmacokinet. 2004;43:1167–78.
Beal SL, Boeckman AJ, Sheiner LB. NONMEM user guides. (1988).
Duffull SB, Denman N, Eccleston J, Kimko H. WinPOPT User Guide Version 1.2. (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Hasselt, J.G.C., Green, B. & Morrish, G.A. Leveraging Physiological Data from Literature into a Pharmacokinetic Model to Support Informative Clinical Study Design in Pregnant Women. Pharm Res 29, 1609–1617 (2012). https://doi.org/10.1007/s11095-012-0671-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0671-2