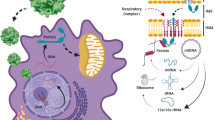
ABSTRACT
Protein therapy is considered an alternative approach to gene therapy for treatment of genetic-metabolic disorders. Human protein therapeutics (PTs), developed via recombinant DNA technology and used for the treatment of these illnesses, act upon membrane-bound receptors to achieve their pharmacological response. On the contrary, proteins that normally act inside the cells cannot be developed as PTs in the conventional way, since they are not able to “cross” the plasma membrane. Furthermore, in mitochondrial disorders, attributed either to depleted or malfunctioned mitochondrial proteins, PTs should also have to reach the subcellular mitochondria to exert their therapeutic potential. Nowadays, there is no effective therapy for mitochondrial disorders. The development of PTs, however, via the Protein Transduction Domain (PTD) technology offered new opportunities for the deliberate delivery of human recombinant proteins inside eukaryotic subcellular organelles. To this end, mitochondrial disorders could be clinically encountered with the delivery of human mitochondrial proteins (engineered via recombinant DNA and PTD technologies) at specific intramitochondrial sites to exert their function. Overall, PTD-mediated Protein Replacement Therapy emerges as a suitable model system for the therapeutic approach for mitochondrial disorders.


Similar content being viewed by others
Abbreviations
- aa:
-
amino acids
- CDS:
-
coding sequence
- CNS:
-
central nervous system
- COX:
-
Cytochrome c Oxidase
- E. coli :
-
Escherichia coli
- ERT:
-
Enzyme Replacement Therapy
- FDA:
-
Food and Drug Administration
- GFP:
-
Green Fluorescent Protein
- GMOs:
-
genetically modified organisms
- IBs:
-
inclusion bodies
- IMS:
-
intermembrane space
- IPTG:
-
isopropyl-beta-D-thiogalactopyranoside
- L:
-
N-terminal Leader Peptide
- LAD:
-
Lipoamide Dehydrogenase
- mab:
-
monoclonal antibody
- MPP:
-
Mitochondrial Processing Peptidase
- mtDNA:
-
mitochondrial DNA
- MTS:
-
Mitochondrial Targeting Signal Peptide
- ΝABs:
-
Neutralizing Antibodies
- nDNA:
-
muclear DNA
- OXPHOS:
-
Oxidative Phosphorylation System
- PDHC:
-
Pyruvate Dehydrogenase Complex
- PEG:
-
poly(ethylene glycol)
- PRT:
-
Protein Replacement Therapy
- PTD:
-
Protein Transduction Domain
- PTs:
-
Protein Therapeutics
- ROS:
-
Reactive Oxygen Species
- TAT:
-
a Protein Transduction Domain
- TCA:
-
Tricarboxylic acid cycle
- TFAM:
-
Mitochondrial Transcription Factor A
REFERENCES
Betz UA, Farquhar R, Ziegelbauer K. Genomics: success or failure to deliver drug targets? Curr Opin Chem Biol. 2005;9(4):387–91.
Banting FG et al. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12(3):141–6.
Villa-Komaroff L et al. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978;75(8):3727–31.
Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39.
Clarke JB. Mechanisms of adverse drug reactions to biologics. Handb Exp Pharmacol. 2010;196:453–74.
Mossalam M, Dixon AS, Lim CS. Controlling subcellular delivery to optimize therapeutic effect. Ther Deliv. 2010;1(1):169–93.
Mokranjac D, Neupert W. Thirty years of protein translocation into mitochondria: unexpectedly complex and still puzzling. Biochim Biophys Acta. 2009;1793(1):33–41.
McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16(14):R551–60.
Schatz G. The magic garden. Annu Rev Biochem. 2007;76:673–8.
Yasukawa K et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2(84):ra47.
DiMauro S. Mitochondrial DNA medicine. Biosci Rep. 2007;27(1–3):5–9.
Zimmer C. Origins. On the origin of eukaryotes. Science. 2009;325(5941):666–8.
Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet. 2001;2(5):342–52.
McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease—its impact, etiology, and pathology. Curr Top Dev Biol. 2007;77:113–55.
Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348.
Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283(5402):689–92.
Chen H, Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–76.
Giles RE et al. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980;77(11):6715–9.
Chinnery PF, Schon EA. Mitochondria. J Neurol Neurosurg Psychiatry. 2003;74(9):1188–99.
Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet. 2008;40(12):1484–8.
Dimauro S. A history of mitochondrial diseases. J Inherit Metab Dis. 2011;34(2):261–76
Schwartz M, Vissing J. Paternal inheritance of mitochondrial DNA. N Engl J Med. 2002;347(8):576–80.
Enns GM. The contribution of mitochondria to common disorders. Mol Genet Metab. 2003;80(1–2):11–26.
Tatsuta T. Protein quality control in mitochondria. J Biochem. 2009;146(4):455–61.
Diaz F. Cytochrome c oxidase deficiency: patients and animal models. Biochim Biophys Acta. 2010;1802(1):100–10.
DiMauro S, Hirano M, Schon EA. Approaches to the treatment of mitochondrial diseases. Muscle Nerve. 2006;34(3):265–83.
Chacinska A et al. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138(4):628–44.
Lee CM et al. The DNA helicase, Hmi1p, is transported into mitochondria by a C-terminal cleavable targeting signal. J Biol Chem. 1999;274(30):20937–42.
Fukui H et al. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104(35):14163–8.
Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18(4):165–73.
Brown GK et al. Pyruvate dehydrogenase deficiency. J Med Genet. 1994;31(11):875–9.
Rotig A. Genetic bases of mitochondrial respiratory chain disorders. Diabetes Metab. 2010;36(2):97–107.
Barrientos A et al. Cytochrome oxidase in health and disease. Gene. 2002;286(1):53–63.
Fontanesi F, Soto IC, Barrientos A. Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life. 2008;60(9):557–68.
Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283(5407):1488–93.
DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. Am J Med Genet. 2001;106(1):18–26.
Massa V et al. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am J Hum Genet. 2008;82(6):1281–9.
Mootha VK et al. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc Natl Acad Sci U S A. 2003;100(2):605–10.
Ghezzi D et al. FASTKD2 nonsense mutation in an infantile mitochondrial encephalomyopathy associated with cytochrome c oxidase deficiency. Am J Hum Genet. 2008;83(3):415–23.
Weraarpachai W et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat Genet. 2009;41(7):833–7.
Zhu Z et al. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat Genet. 1998;20(4):337–43.
Papadopoulou LC et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23(3):333–7.
Valnot I et al. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am J Hum Genet. 2000;67(5):1104–9.
Valnot I et al. A mutation in the human heme A:farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum Mol Genet. 2000;9(8):1245–9.
Antonicka H et al. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am J Hum Genet. 2003;72(1):101–14.
Foltopoulou PF et al. Human recombinant mutated forms of the mitochondrial COX assembly Sco2 protein differ from wild-type in physical state and copper binding capacity. Mol Genet Metab. 2004;81(3):225–36.
Koene S, Smeitink J. Mitochondrial medicine: entering the era of treatment. J Intern Med. 2009;265(2):193–209.
McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2010;9(8):829–40.
Shokolenko IN et al. The approaches for manipulating mitochondrial proteome. Environ Mol Mutagen. 2010;51(5):451–61.
Wenz T et al. Emerging therapeutic approaches to mitochondrial diseases. Dev Disabil Res Rev. 2010;16(2):219–29.
Yamada Y et al. Mitochondrial drug delivery and mitochondrial disease therapy—an approach to liposome-based delivery targeted to mitochondria. Mitochondrion. 2007;7(1–2):63–71.
Moraes CT. Making the most of what you’ve got: optimizing residual OXPHOS function in mitochondrial diseases. EMBO Mol Med. 2009;1(8–9):357–9.
Acin-Perez R et al. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med. 2009;1(8–9):392–406.
Raffaello A, Rizzuto R. Mitochondrial longevity pathways. Biochim Biophys Acta. 2011;1813(1):260–8.
Schon EA et al. Therapeutic prospects for mitochondrial disease. Trends Mol Med. 2010;16(6):268–76.
Cassidy-Stone A et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204.
Perales-Clemente E et al. Restoration of electron transport without proton pumping in mammalian mitochondria. Proc Natl Acad Sci U S A. 2008;105(48):18735–9.
Tanaka M et al. Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J Biomed Sci. 2002;9(6 Pt 1):534–41.
Chinnery PF et al. Peptide nucleic acid delivery to human mitochondria. Gene Ther. 1999;6(12):1919–28.
Hirano M et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology. 2006;67(8):1458–60.
Bredenoord AL, Pennings G, de Wert G. Ooplasmic and nuclear transfer to prevent mitochondrial DNA disorders: conceptual and normative issues. Hum Reprod Update. 2008;14(6):669–78.
Craven L et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465(7294):82–5.
D’Souza GG, Weissig V. Subcellular targeting: a new frontier for drug-loaded pharmaceutical nanocarriers and the concept of the magic bullet. Expert Opin Drug Deliv. 2009;6(11):1135–48.
Srivastava S, Moraes CT. Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum Mol Genet. 2001;10(26):3093–9.
Smith RA et al. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263(3):709–16.
Weissig V, Torchilin VP. Mitochondriotropic cationic vesicles: a strategy towards mitochondrial gene therapy. Curr Pharm Biotechnol. 2000;1(4):325–46.
Horobin RW, Trapp S, Weissig V. Mitochondriotropics: a review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J Control Release. 2007;121(3):125–36.
Rapoport M et al. TAT-mediated delivery of LAD restores pyruvate dehydrogenase complex activity in the mitochondria of patients with LAD deficiency. Mol Ther. 2008;16(4):691–7.
Foltopoulou PF et al. Intracellular delivery of full length recombinant human mitochondrial L-Sco2 protein into the mitochondria of permanent cell lines and SCO2 deficient patient’s primary cells. Biochim Biophys Acta. 2010;1802(6):497–508.
Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9(6):447–64.
Katsura K et al. Combination therapy with transductive anti-death FNK protein and FK506 ameliorates brain damage with focal transient ischemia in rat. J Neurochem. 2008;106(1):258–70.
Yousif LF et al. Mitochondria-penetrating peptides: sequence effects and model cargo transport. Chembiochem. 2009;10(12):2081–8.
Weissig V et al. DQAsomes: a novel potential drug and gene delivery system made from Dequalinium. Pharm Res. 1998;15(2):334–7.
Yamada Y et al. MITO-Porter: a liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta. 2008;1778(2):423–32.
Toogood PL. Mitochondrial drugs. Curr Opin Chem Biol. 2008;12(4):457–63.
Frantz MC, Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen. 2010;51(5):462–75.
Zhang E et al. Newly developed strategies for multifunctional mitochondria-targeted agents in cancer therapy. Drug Discov Today. 2011;16(3-4):140–6.
Swartz JR. Advances in Escherichia coli production of therapeutic proteins. Curr Opin Biotechnol. 2001;12(2):195–201.
Tsumoto K et al. Role of arginine in protein refolding, solubilization, and purification. Biotechnol Prog. 2004;20(5):1301–8.
Houdebine LM. Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis. 2009;32(2):107–21.
Sodoyer R. Expression systems for the production of recombinant pharmaceuticals. BioDrugs. 2004;18(1):51–62.
Tsiftsoglou AS. Biosimilars: the impact of their heterogeneity on regulatory approval. Nat Rev Drug Discov. 2007;6(3).
Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19(3):411–9.
Walsh G. Post-translational modifications of protein biopharmaceuticals. Drug Discov Today. 2010;15(17–18):773–80.
Hamilton SR et al. Production of complex human glycoproteins in yeast. Science. 2003;301(5637):1244–6.
Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21(5):797–802.
Joralemon MJ, McRae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem Commun (Camb). 2010;46(9):1377–93
Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10(21):1451–8.
Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99(6):2557–75.
Rapoport M, Lorberboum-Galski H. TAT-based drug delivery system—new directions in protein delivery for new hopes? Expert Opin Drug Deliv. 2009;6(5):453–63.
Jahn EM, Schneider CK. How to systematically evaluate immunogenicity of therapeutic proteins—regulatory considerations. N Biotechnol. 2009;25(5):280–6.
Barbosa MD, Celis E. Immunogenicity of protein therapeutics and the interplay between tolerance and antibody responses. Drug Discov Today. 2007;12(15–16):674–81.
McKoy JM et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion. 2008;48(8):1754–62.
Ioannou YA et al. Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am J Hum Genet. 2001;68(1):14–25.
Wolbink GJ, Aarden LA, Dijkmans BA. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol. 2009;21(3):211–5.
Weber CA et al. T cell epitope: friend or foe? Immunogenicity of biologics in context. Adv Drug Deliv Rev. 2009;61(11):965–76.
Carcao M, Lambert T. Prophylaxis in haemophilia with inhibitors: update from international experience. Haemophilia. 2010;16 Suppl 2:16–23.
Descotes J. Immunotoxicity of monoclonal antibodies. MAbs. 2009;1(2):104–11.
Bryson CJ, Jones TD, Baker MP. Prediction of immunogenicity of therapeutic proteins: validity of computational tools. BioDrugs. 2010;24(1):1–8.
Stephens DJ, Pepperkok R. The many ways to cross the plasma membrane. Proc Natl Acad Sci U S A. 2001;98(8):4295–8.
Bickel H, Gerrard J, Hickmans EM. Influence of phenylalanine intake on phenylketonuria. Lancet. 1953;265(6790):812–3.
Deduve C. From cytases to lysosomes. Fed Proc. 1964;23:1045-9.
Kang TS, Stevens RC. Structural aspects of therapeutic enzymes to treat metabolic disorders. Hum Mutat. 2009;30(12):1591–610.
Barton NW et al. Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med. 1991;324(21):1464–70.
Desnick RJ. Enzyme replacement and enhancement therapies for lysosomal diseases. J Inherit Metab Dis. 2004;27(3):385–410.
Goldsmith D, Kuhlmann M, Covic A. Through the looking glass: the protein science of biosimilars. Clin Exp Nephrol. 2007;11(3):191–5.
Belting M, Sandgren S, Wittrup A. Nuclear delivery of macromolecules: barriers and carriers. Adv Drug Deliv Rev. 2005;57(4):505–27.
Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–58.
Boado RJ et al. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102(4):1251–8.
van de Waterbeemd H et al. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J Drug Target. 1998;6(2):151–65.
Brasnjevic I et al. Delivery of peptide and protein drugs over the blood-brain barrier. Prog Neurobiol. 2009;87(4):212–51.
Junutula JR et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32.
Moolten FL, Cooperband SR. Selective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cells. Science. 1970;169(940):68–70.
McCarron PA et al. Antibody conjugates and therapeutic strategies. Mol Interv. 2005;5(6):368–80.
Du H et al. The role of mannosylated enzyme and the mannose receptor in enzyme replacement therapy. Am J Hum Genet. 2005;77(6):1061–74.
Garnacho C et al. Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharmacol Exp Ther. 2008;325(2):400–8.
Francis JW et al. CuZn superoxide dismutase (SOD-1):tetanus toxin fragment C hybrid protein for targeted delivery of SOD-1 to neuronal cells. J Biol Chem. 1995;270(25):15434–42.
Brasseur R, Divita G. Happy birthday cell penetrating peptides: Already 20years. Biochim Biophys Acta. 2010;1798(12):2177–81.
Vives E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?”. J Control Release. 2005;109(1–3):77–85.
Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm Res. 2004;21(3):389–93.
Eguchi A, Dowdy SF. siRNA delivery using peptide transduction domains. Trends Pharmacol Sci. 2009;30(7):341–5.
Hu JW et al. Protein transport in human cells mediated by covalently and noncovalently conjugated arginine-rich intracellular delivery peptides. Peptides. 2009;30(9):1669–78.
Wang YH et al. Arginine-rich intracellular delivery peptides noncovalently transport protein into living cells. Biochem Biophys Res Commun. 2006;346(3):758–67.
van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr Opin Biotechnol. 2011;Apr 11.
Schwarze SR et al. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–72.
Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–93.
Fawell S et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994;91(2):664–8.
Derossi D et al. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269(14):10444–50.
Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157(2):195–206.
Kamada H et al. Creation of novel cell-penetrating peptides for intracellular drug delivery using systematic phage display technology originated from Tat transduction domain. Biol Pharm Bull. 2007;30(2):218–23.
Snyder EL et al. Enhanced targeting and killing of tumor cells expressing the CXC chemokine receptor 4 by transducible anticancer peptides. Cancer Res. 2005;65(23):10646–50.
Green I et al. Protein transduction domains: are they delivering? Trends Pharmacol Sci. 2003;24(5):213–5.
Herce HD, Garcia AE. Cell penetrating peptides: how do they do it? J Biol Phys. 2007;33(5–6):345–56.
Hallbrink M et al. Uptake of cell-penetrating peptides is dependent on peptide-to-cell ratio rather than on peptide concentration. Biochim Biophys Acta. 2004;1667(2):222–8.
Mueller J et al. Comparison of cellular uptake using 22 CPPs in 4 different cell lines. Bioconjug Chem. 2008;19(12):2363–74.
Gump JM, June RK, Dowdy SF. Revised role of glycosaminoglycans in TAT protein transduction domain-mediated cellular transduction. J Biol Chem. 2010;285(2):1500–7.
Gros E et al. A non-covalent peptide-based strategy for protein and peptide nucleic acid transduction. Biochim Biophys Acta. 2006;1758(3):384–93.
Dunkin CM et al. Molecular dynamics studies of transportan 10 (tp10) interacting with a POPC lipid bilayer. J Phys Chem B. 2011;115(5):1188–98.
Tiriveedhi V, Butko P. A fluorescence spectroscopy study on the interactions of the TAT-PTD peptide with model lipid membranes. Biochemistry. 2007;46(12):3888–95.
Sarko D et al. The pharmacokinetics of cell-penetrating peptides. Mol Pharm. 2010;7(6):2224–31.
Banks WA, Robinson SM, Nath A. Permeability of the blood-brain barrier to HIV-1 Tat. Exp Neurol. 2005;193(1):218–27.
Cao G et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22(13):5423–31.
Asoh S et al. Protection against ischemic brain injury by protein therapeutics. Proc Natl Acad Sci U S A. 2002;99(26):17107–12.
Cai B et al. TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp Neurol. 2011;227(1):224–31.
Wang H et al. PEGlated magnetic polymeric liposome anchored with TAT for delivery of drugs across the blood-spinal cord barrier. Biomaterials. 2010;31(25):6589–96.
Kwon YM et al. PTD-modified ATTEMPTS system for enhanced asparaginase therapy: a proof-of-concept investigation. J Control Release. 2008;130(3):252–8.
Dutot L et al. Glycosylated cell-penetrating peptides and their conjugates to a proapoptotic peptide: preparation by click chemistry and cell viability studies. J Chem Biol. 2009;3(2):51–65.
Vocero-Akbani A, Lissy NA, Dowdy SF. Transduction of full-length Tat fusion proteins directly into mammalian cells: analysis of T cell receptor activation-induced cell death. Methods Enzymol. 2000;322:508–21.
Flinterman M et al. Delivery of therapeutic proteins as secretable TAT fusion products. Mol Ther. 2009;17(2):334–42.
Liu BR et al. Cellular internalization of quantum dots noncovalently conjugated with arginine-rich cell-penetrating peptides. J Nanosci Nanotechnol. 2010;10(10):6534–43.
Morris MC et al. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100(4):201–17.
Blobel G, Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975;67(3):835–51.
Davis JR, Kakar M, Lim CS. Controlling protein compartmentalization to overcome disease. Pharm Res. 2007;24(1):17–27.
Vyas PM, Payne RM. TAT opens the door. Mol Ther. 2008;16(4):647–8.
Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272(25):16010–7.
Ryu J et al. Intracellular delivery of p53 fused to the basic domain of HIV-1 Tat. Mol Cells. 2004;17(2):353–9.
Snyder EL, Dowdy SF. Protein/peptide transduction domains: potential to deliver large DNA molecules into cells. Curr Opin Mol Ther. 2001;3(2):147–52.
Yoshikawa T et al. Organelle-targeted delivery of biological macromolecules using the protein transduction domain: potential applications for Peptide aptamer delivery into the nucleus. J Mol Biol. 2008;380(5):777–82.
Zhang XY et al. Cellular uptake and lysosomal delivery of galactocerebrosidase tagged with the HIV Tat protein transduction domain. J Neurochem. 2008;104(4):1055–64.
Arakawa M et al. Transduction of anti-cell death protein FNK protects isolated rat hearts from myocardial infarction induced by ischemia/reperfusion. Life Sci. 2007;80(22):2076–84.
Soane L, Fiskum G. TAT-mediated endocytotic delivery of the loop deletion Bcl-2 protein protects neurons against cell death. J Neurochem. 2005;95(1):230–43.
Sugita T et al. Comparative study on transduction and toxicity of protein transduction domains. Br J Pharmacol. 2008;153(6):1143–52.
Saar K et al. Cell-penetrating peptides: a comparative membrane toxicity study. Anal Biochem. 2005;345(1):55–65.
Jarver P, Mager I, Langel U. In vivo biodistribution and efficacy of peptide mediated delivery. Trends Pharmacol Sci. 2010;31(11):528–35.
Kilk K et al. Analysis of in vitro toxicity of five cell-penetrating peptides by metabolic profiling. Toxicology. 2009;265(3):87–95.
Waldeck W et al. Transporter molecules influence the gene expression in HeLa cells. Int J Med Sci. 2009;6(1):18–27.
Eavri R, Lorberboum-Galski H. A novel approach for enzyme replacement therapy. The use of phenylalanine hydroxylase-based fusion proteins for the treatment of phenylketonuria. J Biol Chem. 2007;282(32):23402–9.
Sawant R, Torchilin V. Intracellular transduction using cell-penetrating peptides. Mol Biosyst. 2010;6(4):628–40.
Yukawa H et al. Transduction of cell-penetrating peptides into induced pluripotent stem cells. Cell Transplant. 2010;19(6):901–9.
Johnson RM, Harrison SD, Maclean D. Therapeutic applications of cell-penetrating peptides. Methods Mol Biol. 2010;683:535–51.
Verdurmen WP, Brock R. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol Sci. 2011;32(2):116–24.
Del Gaizo V, MacKenzie JA, Payne RM. Targeting proteins to mitochondria using TAT. Mol Genet Metab. 2003;80(1–2):170–80.
Rayapureddi JP et al. TAT fusion protein transduction into isolated mitochondria is accelerated by sodium channel inhibitors. Biochemistry. 2010;49(44):9470–9.
Del Gaizo V, Payne RM. A novel TAT-mitochondrial signal sequence fusion protein is processed, stays in mitochondria, and crosses the placenta. Mol Ther. 2003;7(6):720–30.
Khan SM, Bennett Jr JP. Development of mitochondrial gene replacement therapy. J Bioenerg Biomembr. 2004;36(4):387–93.
Kaufman BA et al. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol Biol Cell. 2007;18(9):3225–36.
Iyer S et al. Recombinant mitochondrial transcription factor A with N-terminal mitochondrial transduction domain increases respiration and mitochondrial gene expression. Mitochondrion. 2009;9(3):196–203.
Thomas RR et al. Recombinant human mitochondrial transcription factor A stimulates mitochondrial biogenesis and ATP synthesis, improves motor function after MPTP, reduces oxidative stress and increases survival after endotoxin. Mitochondrion. 2010;11:108–18.
Shokolenko IN et al. TAT-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair (Amst). 2005;4(4):511–8.
Rapoport M et al. Successful TAT-mediated enzyme replacement therapy in a mouse model of mitochondrial E3 deficiency. J Mol Med. 2011;89(2):161–70.
Williams JC et al. Crystal structure of human SCO1: implications for redox signaling by a mitochondrial cytochrome c oxidase “assembly” protein. J Biol Chem. 2005;280(15):15202–11.
Matoba S et al. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–3.
Yang H et al. Analysis of mouse models of cytochrome c oxidase deficiency owing to mutations in Sco2. Hum Mol Genet. 2010;19(1):170–80.
Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88(2):223–33.
Futaki S et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276(8):5836–40.
Pooga M et al. Cellular translocation of proteins by transportan. Faseb J. 2001;15(8):1451–3.
ACKNOWLEDGMENTS & DISCLOSURES
We would like to thank Mrs. Elena Kounadi (University Studio Press, Thessaloniki, Greece) for her artistic assistance and Mr. Ioannis D. Bonovolias, PhD student, for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papadopoulou, L.C., Tsiftsoglou, A.S. Transduction of Human Recombinant Proteins into Mitochondria as a Protein Therapeutic Approach for Mitochondrial Disorders. Pharm Res 28, 2639–2656 (2011). https://doi.org/10.1007/s11095-011-0546-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0546-y