Abstract
Purpose
This comparison employs mathematical disease progression models to identify a rat model of arthritis with the least inter-animal variability and features lending to better study designs.
Methods
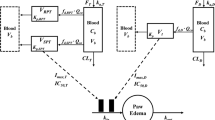
Arthritis was induced with either collagen (CIA) or mycobacterium (AIA) in either Lewis or Dark Agouti (DA) rats. Disease progression was monitored by paw edema and body weight. Models with production, loss, and feedback components were constructed and population analysis using NONMEM software was employed to identify inter-animal variability in the various disease progression parameters.
Results
Onset time was the only parameter different within all four groups (DA–AIA 11.5 days, DA–CIA 16.5 days, Lewis–AIA 11.9 days, Lewis–CIA 13.9 days). The loss-of-edema rate constant was 20% slower in DA (0.362 h−1) than Lewis (0.466 h−1) rats. Most models exhibited peak paw edema 20 days post-induction. Edema in CIA returned to 150% of the initial value after the disease peaked. DA rats displayed more severe overall responses.
Conclusions
No statistical differences between groups were observed for inter-animal variation in disease onset, progression and severity parameters. Onset time varies and should be noted in the design of future studies. DA rats may offer a more dynamic range of edema response than Lewis rats.




Similar content being viewed by others
Abbreviations
- AIA:
-
adjuvant-induced arthritis
- CIA:
-
collagen-induced arthritis
- CV:
-
coefficient of variation
- FOCE:
-
first order conditional estimation
- MVOF:
-
minimum value of the objective function
- PD:
-
pharmacodynamics
- PK:
-
pharmacokinetics
- RA:
-
rheumatoid arthritis
- k out :
-
loss of edema rate constant
- k in :
-
production of edema rate constant
- R deg :
-
loss of production of edema rate constant
- t Onset :
-
disease onset time
- P :
-
parameter
- θ :
-
population mean of P
- η :
-
measure of individual’s deviation from θ
References
R. Holmdahl, J. C. Lorentzen, S. Lu, P. Olofsson, L. Wester, J. Holmberg, and U. Pettersson. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol. Rev. 184:184–202 (2001) doi:10.1034/j.1600-065x.2001.1840117.x.
S. S. Kerwar, N. Bauman, A. L. Oronsky, and A. E. Sloboda. Studies on type II collagen induced polyarthritis in rats. Effect of complement depletion. J. Immunopharmacol. 3:323–337 (1981).
C. M. Pearson. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc. Soc. Exp. Biol. Med. 91:95–101 (1956).
J. M. Stuart, M. A. Cremer, A. S. Townes, and A. H. Kang. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J. Exp. Med. 155:1–16 (1982) doi:10.1084/jem.155.1.1.
J. D. A. Taurog, D. C. Argentieri, and R. A. McReynolds. Adjuvant arthritis. Methods Enzymol. 162:339–355 (1956) doi:10.1016/0076-6879(88)62089-1.
J. R. Ward, and R. S. Jones. Studies on adjuvant-induced polyarthritis in rats. I. Adjuvant composition, route of injection, and removal of depot site. Arthritis Rheum. 5:557–564 (1962) doi:10.1002/art.1780050604.
P. H. Wooley. The usefulness and the limitations of animal models in identifying targets for therapy in arthritis. Best Pract. Res. Clin. Rheumatol. 18:47–58 (2004) doi:10.1016/j.berh.2003.09.007.
S. Akilesh, S. Petkova, T. J. Sproule, D. J. Shaffer, G. J. Christianson, and D. Roopenian. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J. Clin. Invest. 113:1328–1333 (2004).
J. S. Courtenay, M. J. Dallman, A. D. Dayan, A. Martin, and B. Mosedale. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 283:666–668 (1980) doi:10.1038/283666a0.
D. E. Trentham, A. S. Townes, and A. H. Kang. Autoimmunity to type II collagen an experimental model of arthritis. J. Exp. Med. 146:857–868 (1977) doi:10.1084/jem.146.3.857.
O. Kohashi, K. Aihara, A. Ozawa, S. Kotani, and I. Azuma. New model of a synthetic adjuvant, N-acetylmuramyl-l-alanyl-d-isoglutamine- induced arthritis: clinical and histologic studies in athymic nude and euthymic rats. Lab Invest. 47:27–36 (1982).
O. Kohashi, C. M. Pearson, Y. Watanabe, and S. Kotani. Preparation of arthritogenic hydrosoluble peptidoglycans from both arthritogenic and non-arthritogenic bacterial cell walls. Infect. Immun. 16:861–866 (1977).
F. C. Colpaert, T. Meert, P. De Witte, and P. Schmitt. Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sci. 31:67–75 (1982) doi:10.1016/0024-3205(82)90402-7.
R. Holmdahl, C. Vingsbo, V. Malmstrom, L. Jansson, and M. Holmdahl. Chronicity of arthritis induced with homologous type II collagen (CII) in rats is associated with anti-CII B-cell activation. J. Autoimmun. 7:739–752 (1994) doi:10.1006/jaut.1994.1058.
P. Larsson, S. Kleinau, R. Holmdahl, and L. Klareskog. Homologous type II collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis Rheum. 33:693–701 (1990) doi:10.1002/art.1780330512.
C. M. W. Pearson, and F. M. Wood. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis Rheum. 2:440–459 (1959) doi:10.1002/1529-0131(195910)2:5<440::AID-ART1780020510>3.0.CO;2-N.
K. A. Bush, B. W. Kirkham, and J. S. Walker. The in vivo effects of tumour necrosis factor blockade on the early cell mediated immune events and syndrome expression in rat adjuvant arthritis. Clin. Exp. Immunol. 127:423–429 (2002) doi:10.1046/j.1365-2249.2002.01742.x.
D. W. Hunt, L. Corson, H. D. Barker, J. G. Levy, and R. E. Petty. Relationship between collagen-induced and adjuvant arthritis in the Lewis rat. J. Autoimmun. 6:691–700 (1993) doi:10.1006/jaut.1993.1058.
G. S. Panayi, J. S. Lanchbury, and G. H. Kingsley. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 35:729–735 (1992) doi:10.1002/art.1780350702.
S. S. Kerwar, M. E. Englert, R. A. McReynolds, M. J. Landes, J. M. Lloyd, A. L. Oronsky, and F. J. Wilson. Type II collagen-induced arthritis. Studies with purified anticollagen immunoglobulin. Arthritis Rheum. 26:1120–1131 (1983) doi:10.1002/art.1780260910.
I. Rioja, K. A. Bush, J. B. Buckton, M. C. Dickson, and P. F. Life. Joint cytokine quantification in two rodent arthritis models: kinetics of expression, correlation of mRNA and protein levels and response to prednisolone treatment. Clin. Exp. Immunol. 137:65–73 (2004) doi:10.1111/j.1365-2249.2004.02499.x.
E. Larsson, A. Mussener, D. Heinegard, L. Klareskog, and T. Saxne. Increased serum levels of cartilage oligomeric matrix protein and bone sialoprotein in rats with collagen arthritis. Br. J. Rheumatol. 36:1258–1261 (1997) doi:10.1093/rheumatology/36.12.1258.
J. M. Stuart, K. Tomoda, T. J. Yoo, A. S. Townes, and A. H. Kang. Serum transfer of collagen-induced arthritis. II. Identification and localization of autoantibody to type II collagen in donor and recipient rats. Arthritis Rheum. 26:1237–1244 (1983) doi:10.1002/art.1780261011.
K. Takagishi, N. Kaibara, T. Hotokebuchi, C. Arita, M. Morinaga, and K. Arai. Serum transfer of collagen arthritis in congenitally athymic nude rats. J. Immunol. 134:3864–3867 (1985).
P. Wernhoff, C. Unger, E. Bajtner, H. Burkhardt, and R. Holmdahl. Identification of conformation-dependent epitopes and V gene selection in the B cell response to type II collagen in the DA rat. Int. Immunol. 13:909–919 (2001) doi:10.1093/intimm/13.7.909.
J. C. Lorentzen, and L. Klareskog. Comparative susceptibility of DA, LEW, and LEW.1AV1 rats to arthritis induced with different arthritogens: mineral oil, mycobacteria, muramyl dipeptide, avridine and rat collagen type II. Transplant. Proc. 29:1692–1693 (1997) doi:10.1016/S0041-1345(97)00018-3.
C. M. Rubino, E. V. Capparelli, J. S. Bradley, J. L. Blumer, G. L. Kearns, M. Reed, R. F. Jacobs, B. Cirincione, and D. M. Grasela. Population pharmacokinetic model for gatifloxacin in pediatric patients. Antimicrob. Agents Chemother. 51:1246–1252 (2007) doi:10.1128/AAC.00685-06.
U. Wählby, E. N. Jonsson, and M. O. Karlsson. Assessment of actual significance levels for covariate effects in NONMEM. J. Pharmacokinet. Pharmacodyn. 28:231–252 (2001).
M. Dimitrijevic, O. Laban, V. J. Djuric, S. Stanojevic, T. Miletic, V. Kovacevic-Jovanovic, C. Todorovic, and J. Radulovic. Behavior and severity of adjuvant arthritis in four rat strains. Brain Behav. Immun. 15:255–265 (2001) doi:10.1006/brbi.2000.0599.
G. W. Cannon, M. L. Woods, F. Clayton, and M. M. Griffiths. Induction of arthritis in DA rats by incomplete Freund’s adjuvant. J. Rheumatol. 20:7–11 (1993).
S. Carlsen, A. S. Hansson, H. Olsson, D. Heinegard, and R. Holmdahl. Cartilage oligomeric matrix protein (COMP)-induced arthritis in rats. Clin. Exp. Immunol. 114:477–484 (1998) doi:10.1046/j.1365-2249.1998.00739.x.
R. Holmdahl, T. J. Goldschmidt, S. Kleinau, C. Kvick, and R. Jonsson. Arthritis induced in rats with adjuvant oil is a genetically restricted, alpha beta T-cell dependent autoimmune disease. Immunology. 76:197–202 (1992).
S. Kleinau, H. Erlandsson, R. Holmdahl, and L. Klareskog. Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence for an immunological involvement. J. Autoimmun. 4:871–880 (1991) doi:10.1016/0896-8411(91)90050-M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Earp, J.C., DuBois, D.C., Almon, R.R. et al. Quantitative Dynamic Models of Arthritis Progression in the Rat. Pharm Res 26, 196–203 (2009). https://doi.org/10.1007/s11095-008-9711-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9711-3