Abstract
Purpose
Tubulointerstitial fibrosis is a final common pathway to end-stage chronic kidney diseases, which are characterized by elevated renal angiotensin II (AngII) production. This peptide participates in kidney damage inducing fibrosis and epithelial mesenchymal transition (EMT). Our aim was to describe potential therapeutic targets in AngII-induced EMT, investigating the blockade of different intracellular pathways.
Methods
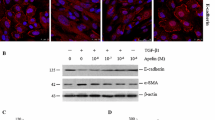
Studies were done in human tubular epithelial cells (HK2 cell line), evaluating changes in phenotype and EMT markers (Western blot and immunofluorescence).
Results
Treatment of HK2 cells with AngII for 3 days caused transdifferentiation into myofibroblast-like cells. The blockade of MAPKs cascade, using specific inhibitors of p38 (SB203580), extracellular signal-regulated kinase1/2 (ERK; PD98059) and Jun N-terminal kinase (JNK) (SP600125), diminished AngII-induced EMT. The blockade of RhoA/ROCK pathway, by transfection of a RhoA dominant-negative vector or by ROCK inhibition with Y-27632 or fasudil, inhibited EMT caused by AngII. Connective tissue growth factor (CTGF) is a downstream mediator of AngII-induced EMT. MAPKs and ROCK inhibitors blocked CTGF overexpression induced by AngII. HMG-CoA reductase inhibitors, although blocked AngII-mediated kinases activation, only partially diminished EMT and did not regulate CTGF.
Conclusions
These data suggest a potential therapeutic use of kinase inhibitors in renal fibrosis.












Similar content being viewed by others
Abbreviations
- AngII:
-
angiotensin II
- AT:
-
angiotensin receptors
- CTGF:
-
connective tissue growth factor
- EMT:
-
epithelial mesenchymal transition
- ERK:
-
extracellular signal-regulated kinase1/2
- FBS:
-
fetal bovine serum
- HK2:
-
human tubular epithelial cell line
- HMG-CoA reductase:
-
3-hydroxy-3-methylglutaryl coenzyme A reductase
- JNK:
-
Jun N-terminal
- MAPK:
-
mitogen activated kinases
- ROCK:
-
rho-kinase
- TGF-β:
-
transforming growth factor-beta
- VSMC:
-
vascular smooth muscle cells
References
G. Wolf, and E. Ritz. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 67:799–812 (2005). doi:10.1111/j.1523-1755.2005.00145.x.
E. G. Neilson. Mechanisms of disease: fibroblasts—a new look at an old problem. Nat Clin Pract Nephrol. 2:101–118 (2006). doi:10.1038/ncpneph0093.
G. Wolf. Renal injury due to rennin–angiotensin–aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 70:1914–1919 (2006).
M. Ruiz-Ortega, M. Ruperez, V. Esteban, J. Rodriguez-Vita, E. Sanchez-Lopez, G. Carvajal, and J. Egido. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial. Transplant. 21:16–20 (2006). doi:10.1093/ndt/gfi265.
M. Ruiz-Ortega, J. Rodríguez-Vita, E. Sanchez-Lopez, G. Carvajal, and J. Egido. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res. 74:196–206 (2007). doi:10.1016/j.cardiores.2007.02.008.
G. Carvajal-Gonzalez, J. Rodriguez-Vita, R. Rodrigues-Diez, E. Sanchez-Lopez, M. Ruperez, C. Cartier, V. Esteban, A. Ortiz, J Egido, S. Mezzano, and M. Ruiz-Ortega. Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int in press (2008) doi:10.1038/ki.2008.213.
A. V. Bakin, C. Rinehart, A. K. Tomlinson, and C. L. Arteaga. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 115:3193–3206 (2002).
V. Ellenrieder, S. F. Hendler, W. Boeck, T. Seufferlein, A. Menke, C. Ruhland, G. Adler, and T. M. Gress. Transforming growth factor beta1 treatment leads to an epithelial–mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 61:4222–4228 (2001).
S. Patel, K. I. Takagi, J. Suzuki, A. Imaizumi, T. Kimura, R. M. Mason, T. Kamimura, and Z. Zhang. RhoGTPase activation is a key step in renal epithelial mesenchymal transdifferentiation. J. Am. Soc. Nephrol. 16:1977–1984 (2005). doi:10.1681/ASN.2004110943.
N. A. Bhowmick, M. Ghiassi, A. Bakin, M. Aakre, C. A. Lundquist, M. E. Engel, C. L. Arteaga, and H. L. Moses. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell. 12:27–36 (2001).
J. K. Liao. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am. J. Cardiol. 96:24F–33F (2005). doi:10.1016/j.amjcard.2005.06.009.
J. Tuñón, J. L. Martín-Ventura, L. M. Blanco-Colio, and J. Egido. Mechanisms of action of statins in stroke. Expert Opin. Ther. Targets. 11(3):273–278 (2007). doi:10.1517/14728222.11.3.273.
M. Rupérez, R. Rodrigues-Díez, L. M. Blanco-Colio, E. Sánchez-López, J. Rodríguez-Vita, V. Esteban, G. Carvajal, J. J. Plaza, J. Egido, and M. Ruiz-Ortega. HMG-CoA reductase inhibitors decrease angiotensin II-induced vascular fibrosis: role of RhoA/ROCK and MAPK pathways. Hypertension. 50:377–383 (2007). doi:10.1161/HYPERTENSIONAHA.107.091264.
G. D’Amico. Statins and renal diseases: from primary prevention to renal replacement therapy. J. Am. Soc. Nephrol. 17(4 Suppl 2):S148–52 (2006). doi:10.1681/ASN.2005121341.
A. Gianella, E. Nobili, M. Abbate, C. Zoja, P. Gelosa, L. Mussoni, S. Bellosta, M. Canavesi, D. Rottoli, U. Guerrini, M. Brioschi, C. Banfi, E. Tremoli, G. Remuzzi, and L. Sironi. Rosuvastatin treatment prevents progressive kidney inflammation and fibrosis in stroke-prone rats. Am. J. Pathol. 170:1165–1177 (2007). doi:10.2353/ajpath.2007.060882.
S. Patel, R. M. Mason, J. Suzuki, A. Imaizumi, T. Kamimura, and Z. Zhang. Inhibitory effect of statins on renal epithelial-to-mesenchymal transition. Am. J. Nephrol. 26:381–387 (2006). doi:10.1159/000094780.
J. L. Wei, C. Y. Ma, Y. D. Zhang, and Y. Li. Synergistic effects of pravastatin and pioglitazone in renal tubular epithelial cells induced by transforming growth factor-beta1. Cell Biol. Int. 31:451–458 (2007). doi:10.1016/j.cellbi.2006.11.008.
L. Chen, B. C. Liu, X. L. Zhang, J. D. Zhang, H. Liu, and M. X. Li. Influence of connective tissue growth factor antisense oligonucleotide on angiotensin II-induced epithelial mesenchymal transition in HK2 cells. Acta Pharmacologica Sinica. 27:1029–1036 (2007). doi:10.1111/j.1745-7254.2006.00344.x.
D. T. Dudley, L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U.S.A. 92:7686–7689 (1995). doi:10.1073/pnas.92.17.7686.
J. C. Lee, J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, J. E. Strickler, M. M. McLaughlin, I. R. Siemens, S. M. Fisher, G. P. Livi, J. R. White, J. L. Adams, and P. R. Young. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 372:739–746 (1994). doi:10.1038/372739a0.
C. Zhang, X. Meng, Z. Zhu, X. Yang, and A. Deng. Role of connective tissue growth factor in renal tubular epithelial-myofibroblast transdifferentiation and extracellular matrix accumulation in vitro. Life Sci. 75:367–379 (2004). doi:10.1016/j.lfs.2004.02.005.
E. Gore-Hyer, D. Shegogue, M. Markiewicz, S. Lo, D. Hazen-Martin, E. L. Greene, G. Grotendorst, and M. Trojanowska. TGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am. J. Physiol. Renal Physiol. 283:F707–F716 (2002).
K. S. Frazier, A. Paredes, P. Dube, and E. Styer. Connective tissue growth factor expression in the rat remnant kidney model and association with tubular epithelial cells undergoing transdifferentiation. Vet. Pathol. 37:328–335 (2000). doi:10.1354/vp.37-4-328.
M. Ruperez, M. Ruiz-Ortega, V. Esteban, O. Lorenzo, S. Mezzano, J. J. Plaza, and J. Egido. Angiotensin II increases connective tissue growth factor in the kidney. Am. J. Pathol. 163:1937–1947 (2003).
N. A. Wahab, B. S. Weston, and R. M. Mason. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J. Am. Soc. Nephrol. 16:340–351 (2005). doi:10.1681/ASN.2003100905.
M. Rupérez, O. Lorenzo, L. M. Blanco-Colio, V. Esteban, J. Egido, and M. Ruiz-Ortega. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 108:1499–1505 (2003). doi:10.1161/01.CIR.0000089129.51288.BA.
E. Sánchez-López, J. Rodriguez-Vita, C. Cartier, M. Rupérez, V. Esteban, G. Carvajal, R. Rodrigues-Díez, J. J. Plaza, J. Egido, and M. Ruiz-Ortega. Inhibitory effect of interleukin-1 on angiotensin II-induced connective tissue growth factor and type IV collagen production in cultured mesangial cells. Am. J. Physiol. Renal Physiol. 294:F149–60 (2008). doi:10.1152/ajprenal.00129.2007.
B. Liu, J. Yu, L. Taylor, X. Zhou, and P. Polgar. Microarray and phosphokinase screenings leading to studies on ERK and JNK regulation of connective tissue growth factor expression by angiotensin II 1a and bradykinin B2 receptors in Rat1 fibroblasts. J. Cell. Biochem. 97:1104–1120 (2006). doi:10.1002/jcb.20709.
A. Moustakas, and C. H. Heldin. Non-Smad TGF-beta signals. J. Cell Sci. 118:3573–3584 (2005). doi:10.1242/jcs.02554.
A. V. Bakin, C. Rinehart, A. K. Tomlinson, and C. L. Arteaga. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 115:3193–3206 (2002).
V. Ellenrieder, S. F. Hendler, W. Boeck, T. Seufferlein, A. Menke, C. Ruhland, G. Adler, and T. M. Gress. Transforming growth factor beta1 treatment leads to an epithelial–mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 61:4222–4228 (2001).
D. Y. Rhyu, Y. Yang, H. Ha, G. T. Lee, J. S. Song, S. T. Uh, and H. B. Lee. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial–mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 16:667–675 (2005). doi:10.1681/ASN.2004050425.
T. Masaki, C. Stambe, P. A. Hill, J. Dowling, R. C. Atkins, and D. J. Nikolic-Paterson. Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. J. Am. Soc. Nephrol. 15:1835–1843 (2004). doi:10.1097/01.ASN.0000130623.66271.67.
C. Stambe, D. J. Nikolic-Paterson, P. A. Hill, J. Dowling, and R. C. Atkins. p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J. Am. Soc. Nephrol. 15:326–336 (2004). doi:10.1097/01.ASN.0000108520.63445.E0.
C. Stambe, R. C. Atkins, P. A. Hill, and D. J. Nikolic-Paterson. Activation and cellular localization of the p38 and JNK MAPK pathways in rat crescentic glomerulonephritis. Kidney Int. 64:2121–2132 (2003). doi:10.1046/j.1523-1755.2003.00324.x.
R. S. Flanc, F. Y. Ma, G. H. Tesch, Y. Han, R. C. Atkins, B. L. Bennett, G. C. Friedman, J. H. Fan, and D. J. Nikolic-Paterson. A pathogenic role for JNK signaling in experimental anti-GBM glomerulonephritis. Kidney Int. 72:698–708 (2007). doi:10.1038/sj.ki.5002404.
Y. Wang, H. X. Ji, S. H. Xing, D. S. Pei, and Q. H. Guan. SP600125, a selective JNK inhibitor, protects ischemic renal injury via suppressing the extrinsic pathways of apoptosis. Life Sci. 80:2067–2075 (2007). doi:10.1016/j.lfs.2007.03.010.
F. Y. Ma, R. S. Flanc, G. H. Tesch, Y. Han, R. C. Atkins, B. L. Bennett, G. C. Friedman, J. H. Fan, and D. J. Nikolic-Paterson. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J. Am. Soc. Nephrol. 18:472–484 (2007). doi:10.1681/ASN.2006060604.
D. Bokemeyer, D. Panek, H. J. Kramer, M. Lindemann, M. Kitahara, P. Boor, D. Kerjaschki, J. M. Trzaskos, J. Floege, and T. Ostendorf. In vivo identification of the mitogen-activated protein kinase cascade as a central pathogenic pathway in experimental mesangioproliferative glomerulonephritis. J. Am .Soc. Nephrol. 13:1473–1480 (2002). doi:10.1097/01.ASN.0000017576.50319.AC.
A. Tojo, M. L. Onozato, N. Kobayashi, A. Goto, H. Matsuoka, and T. Fujita. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J. Hypertens. 23:165–174 (2005). doi:10.1097/00004872-200501000-00027.
S. S. Nerurkar, A. R. Olzinski, K. S. Frazier, R. C. Mirabile, S. P. O’Brien, J. Jing, D. Rajagopalan, T. L. Yue, and R. N. Willette. P38 MAPK inhibitors suppress biomarkers of hypertension end-organ damage, osteopontin and plasminogen activator inhibitor-1. Biomarkers. 12(1):87–112 (2007). doi:10.1080/13547500600944930.
M. H. de Borst, M. M. van Timmeren, V. S. Vaidya, R. A. de Boer, M. B. van Dalen, A. B. Kramer, T. A. Schuurs, J. V. Bonventre, G. Navis, and H. van Goor. Induction of kidney injury molecule-1 in homozygous Ren2 rats is attenuated by blockade of the rennin–angiotensin system or p38 MAP kinase. Am. J. Physiol. Renal Physiol. 292(1):F313–20 (2007). doi:10.1152/ajprenal.00180.2006.
M. H. de Borst, G. Navis, R. A. de Boer, S. Huitema, L. M. Vis, W. H. van Gilst, and H. van Goor. Specific MAP-kinase blockade protects against renal damage in homozygous TGR(mRen2)27 rats. Lab. Invest. 83(12):1761–70 (2003). doi:10.1097/01.LAB.0000101731.11015.F6.
J. K. Park, R. Fischer, R. Dechend, E. Shagdarsuren, A. Gapeljuk, M. Wellner, S. Meiners, P. Gratze, N. Al-Saadi, S. Feldt, A. Fiebeler, J. B. Madwed, A. Schirdewan, H. Haller, F. C. Luft, and D. N. Muller. p38 mitogen-activated protein kinase inhibition ameliorates angiotensin II-induced target organ damage. Hypertension. 49:481–489 (2007). doi:10.1161/01.HYP.0000256831.33459.ea.
J. Prakash, M. Sandovici, V. Saluja, M. Lacombe, R. Q. Schaapveld, M. H. de Borst, H. van Goor, R. H. Henning, J. H. Proost, F. Moolenaar, G. Këri, D. K. Meijer, K. Poelstra, and R. J. Kok. Intracellular delivery of the p38 mitogen-activated protein kinase inhibitor SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole] in renal tubular cells: a novel strategy to treat renal fibrosis. Pharmacol. Exp. Ther. 319(1):8–19 (2006). doi:10.1124/jpet.106.106054.
D. L. Lee, R. C. Webb, and L. Jin. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 44:796–799 (2004). doi:10.1161/01.HYP.0000148303.98066.ab.
S. Patel, K. I. Takagi, J. Suzuki, A. Imaizumi, T. Kimura, R. M. Mason, T. Kamimura, and Z. Zhang. RhoGTPase activation is a key step in renal epithelial mesenchymal transdifferentiation. J. Am. Soc. Nephrol. 16:1977–1984 (2005). doi:10.1681/ASN.2004110943.
N. A. Bhowmick, M. Ghiassi, A. Bakin, M. Aakre, C. A. Lundquist, M. E. Engel, C. L. Arteaga, and H. L. Moses. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell. 12:27–36 (2001).
B. Ozdamar, R. Bose, M. Barrios-Rodiles, H. R. Wang, Y. Zhang, and J. L. Wrana. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 307:1603–1609 (2005). doi:10.1126/science.1105718.
M. Ruperez, E. Sanchez-Lopez, L. M. Blanco-Colio, V. Esteban, J. Rodriguez-Vita, J. J. Plaza, J. Egido, and M. Ruiz-Ortega. The rho-kinase pathway regulates angiotensin II-induced renal damage. Kidney Int. 68:S39–S45 (2005). doi:10.1111/j.1523-1755.2005.09908.x.
C. Kataoka, K. Egashira, S. Inoue, M. Takemoto, W. Ni, M. Koyanagi, S. Kitamoto, M. Usui, K. Kaibuchi, H. Shimokawa, and A. Takeshita. Important role of rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 39:245–250 (2002). doi:10.1161/hy0202.103271.
M. M. Fretz, J. Prakash, M. E. M. Dolman, M. Lacombe, M. de Borst, H. van Goor, K. Poelstra, and R. J. Kok. Renal delivery of kinase inhibitors ameliorates experimental inflammation and fibrosis. J. Am. Soc. Nephrol. 18:100A (2007).
B. Perbal. CCN proteins: multifunctional signalling regulators. Lancet. 363:62–64 (2004). doi:10.1016/S0140-6736(03)15172-0.
M. Guha, Z. G. Xu, D. Tung, L. Lanting, and R. Natarajan. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 21:3355–3368 (2007). doi:10.1096/fj.06-6713com.
J. Heusinger-Ribeiro, M. Eberlein, N. A. Wahab, and M. Goppelt-Struebe. Expression of connective tissue growth factor in human renal fibroblasts: regulatory roles of RhoA and cAMP. J. Am. Soc. Nephrol. 12:1853–1851 (2001).
D. Iwanciw, M. Rehm, M. Porst, and M. Goppelt-Struebe. Induction of connective tissue growth factor by angiotensin II: integration of signaling pathways. Arterioscler. Thromb. Vasc. Biol. 23:1782–1787 (2003). doi:10.1161/01.ATV.0000092913.60428.E6.
Z. Huang, L. Taylor, B. Liu, J. Yu, and P. Polgar. Modulation by bradykinin of angiotensin type 1 receptor-evoked RhoA activation of connective tissue growth factor expression in human lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 290:L1291–L1299 (2006). doi:10.1152/ajplung.00443.2005.
M. Buemi, F. Floccari, L. Nostro, S. Campo, C. Caccamo, A. Sturiale, C. Aloisi, M. S. Giacobbe, and N. Frisina. Statins in the prevention of cardiovascular events in patients with renal failure. Cardiovasc. Hematol. Disord. Drug Targets. 7:7–13 (2007).
L. F. Fried, T. J. Orchard, and B. L. Kasiske. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 59:260–269 (2001). doi:10.1046/j.1523-1755.2001.00487.x.
S. C. Tang, J. C. Leung, L. Y. Chan, A. A. Eddy, and K. N. Lai. Angiotensin converting enzyme inhibitor but not angiotensin receptor blockade or statin ameliorates murine adriamycin nephropathy. Kidney Int. 73:288–299 (2007).
J. M. Vieira, E. Mantovani, L. T. Rodrigues, H. Dellê, I. L. Noronha, C. K. Fujihara, and R. Zatz. Simvastatin attenuates renal inflammation, tubular transdifferentiation and interstitial fibrosis in rats with unilateral ureteral obstruction. Nephrol. Dial. Transplant. 20:1582–1591 (2005). doi:10.1093/ndt/gfh859.
K. L. Watts, and M. A. Spiteri. Connective tissue growth factor expression and induction by transforming growth factor-beta is abrogated by simvastatin via a Rho signaling mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 287:L1323–L1332 (2004). doi:10.1152/ajplung.00447.2003.
M. Eberlein, J. Heusinger-Ribeiro, and M. Goppelt-Struebe. Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins). Br. J Pharmacol. 133:1172–1180 (2001). doi:10.1038/sj.bjp.0704173.
M. Goppelt-Struebe, A. Hahn, D. Iwanciw, M. Rehm, and B. Banas. Regulation of connective tissue growth factor (ccn2; ctgf) gene expression in human mesangial cells: modulation by HMG CoA reductase inhibitors (statins). Mol. Pathol. 54:176–179 (2001). doi:10.1136/mp.54.3.176.
Acknowledgment
This work has been supported by grants from SAF 2005-03378 of the Ministerio de Educación y Ciencia, Sociedad Española de Nefrologia, Red temática de Investigación Renal, REDINREN (ISCIII-RETIC RD06/0016/0004) from the Instituto de Salud Carlos III from Ministerio de Sanidad y Consumo, EU project (DIALOK, LSHB-CT-2007-036644), PCI Iberoamerica and FONDECYT, Chile (1080083). E.S-L, J. R-V. are fellows of FIS. G.C. is a fellow of Fundación Carolina and Fundación Iñigo Alvarez de Toledo. We want to thank Ma Mar Gonzalez Garcia-Parreño and Sandra López León for their technical help. There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Raquel Rodrigues-Díez and Gisselle Carvajal-González contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Rodrigues-Díez, R., Carvajal-González, G., Sánchez-López, E. et al. Pharmacological Modulation of Epithelial Mesenchymal Transition Caused by Angiotensin II. Role of ROCK and MAPK Pathways. Pharm Res 25, 2447–2461 (2008). https://doi.org/10.1007/s11095-008-9636-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9636-x