Abstract
Purpose
Elevated expression of the ABC transporters P-glycoprotein (P-gp), and breast cancer resistance protein (BCRP) seems to correlate with multidrug resistance of cancer cells. In this study we investigated the effect of COX inhibitors in modulating P-gp and BCRP expression and P-gp activity in Caco-2 cells.
Methods
mRNA and protein expression of MDR1 and BCRP were evaluated by real time PCR and western blot respectively. The activity of P-gp was measured by intracellular accumulation of rhodamine123 or 3H-Digoxin.
Results
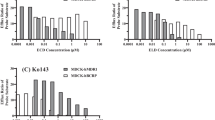
The chronic exposure of Caco-2 to indomethacin heptyl ester (indo HE) (0.4 μM) or nimesulide (10 μM) (selective COX-2 inhibitors) and naproxen (6 μM) (non selective inhibitor COX-1/COX-2) significantly decreased the expression and activity of P-gp. In contrast, the acute treatment by nimesulide and naproxen did not modify these parameters while indo HE treatment (48–72 h) caused a protein decrease and a functional inhibition of P-gp. Unexpectedly, the short-term treatment with naproxen induced an important increase of BCRP expression, but this induction was lost after long-term treatment. No modification of BCRP expression was observed after indo HE or nimesulide treatment.
Conclusion
Our observations suggest a possible down regulation of P-gp by COX inhibitors, which may enhance the accumulation of chemotherapy agents.














Similar content being viewed by others
References
V. Lingand, and L. H. Thompson. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J. Cell. Physiol. 83:103–116 (1974).
F. Thiebaut, T. Tsuruo, H. Hamada, M. M. Gottesman, I. Pastan, and M. C. Willingham. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA. 84:7735–7738 (1987).
M. F. Fromm. P-glycoprotein: a defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int. J. Clin. Pharmacol. Ther. 38:69–74 (2000).
W. L. Smithand, and D. L. Dewitt. Prostaglandin endoperoxide H synthases-1 and -2. Adv. Immunol. 62:167–215 (1996).
G. P. O'Neilland, and A. W. Ford-Hutchinson. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 330:156–60 (1993).
C. C. Chan, S. Boyce, C. Brideau, A. W. Ford-Hutchinson, R. Gordon, D. Guay, R. G. Hill, C. S. Li, J. Mancini, M. Penneton et al. Pharmacology of a selective cyclooxygenase-2 inhibitor, L-745,337: a novel nonsteroidal anti-inflammatory agent with an ulcerogenic sparing effect in rat and nonhuman primate stomach. J. Pharmacol. Exp. Ther. 274:1531–1537 (1995).
T. Tanioka, Y. Nakatani, T. Kobayashi, M. Tsujimoto, S. Oh-ishi, M. Murakami, and I. Kudo. Regulation of cytosolic prostaglandin E2 synthase by 90-kDa heat shock protein. Biochem. Biophys. Res. Commun. 303:1018–1023 (2003).
R. N. Dubois, S. B. Abramson, L. Crofford, R. A. Gupta, L. S. Simon, L. B. Van De Putte, and P. E. Lipsky. Cyclooxygenase in biology and disease. FASEB J. 12:1063–1073 (1998).
C. E. Trebino, J. L. Stock, C. P. Gibbons, B. M. Naiman, T. S. Wachtmann, J. P. Umland, K. Pandher, J. M. Lapointe, S. Saha, M. L. Roach, D. Carter, N. A. Thomas, B. A. Durtschi, J. D. McNeish, J. E. Hambor, P. J. Jakobsson, T. J. Carty, J. R. Perez, and L. P. Audoly. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. USA. 100:9044–9049 (2003).
C. Patrono, P. Patrignani, and L. A. Garcia Rodriguez. Cyclooxygenase-selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outs. J. Clin. Invest. 108:7–13 (2001).
J. A. Mitchelland, and T. D. Warner. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 128:1121–32 (1999).
I. I. Singer II, D. W. Kawka, S. Schloemann, T. Tessner, T. Riehl, and W. F. Stenson. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 115:297–306 (1998).
C. E. Eberhart, R. J. Coffey, A. Radhika, F. M. Giardiello, S. Ferrenbach, and R. N. DuBois. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 107:1183–8 (1994).
H. Sheng, J. Shao, J. D. Morrow, R. D. Beauchamp, and R. N. DuBois. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 58:362–366 (1998).
M. Tsujii, S. Kawano, S. Tsuji, H. Sawaoka, M. Hori, and R. N. DuBois. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 93:705–716 (1998).
N. Arber, C. J. Eagle, J. Spicak, I. Racz, P. Dite, J. Hajer, M. Zavoral, M. J. Lechuga, P. Gerletti, J. Tang, R. B. Rosenstein, K. Macdonald, P. Bhadra, R. Fowler, J. Wittes, A. G. Zauber, S. D. Solomon, and B. Levin. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 355:885–95 (2006).
Y. Goldberg, Nassif, II, A. Pittas, L. L. Tsai, B. D. Dynlacht, B. Rigas, and S. J. Shiff. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene 12:893–901 (1996).
J. Shao, T. Fujiwara, Y. Kadowaki, T. Fukazawa, T. Waku, T. Itoshima, T. Yamatsuji, M. Nishizaki, J. A. Roth, and N. Tanaka. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 19:726–36 (2000).
S. Hashitani, M. Urade, N. Nishimura, T. Maeda, K. Takaoka, K. Noguchi, and K. Sakurai. Apoptosis induction and enhancement of cytotoxicity of anticancer drugs by celecoxib, a selective cyclooxygenase-2 inhibitor, in human head and neck carcinoma cell lines. Int. J. Oncol. 23:665–672 (2003).
J. L. Masferrer, K. M. Leahy, A. T. Koki, B. S. Zweifel, S. L. Settle, B. M. Woerner, D. A. Edwards, A. G. Flickinger, R. J. Moore, and K. Seibert. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 60:1306–1311 (2000).
M. K. Jones, H. Wang, B. M. Peskar, E. Levin, R. M. Itani, I. J. Sarfeh, and A. S. Tarnawski. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat. Med. 5:1418–1423 (1999).
V. A. Patel, M. J. Dunn, and A. Sorokin. Regulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2. J. Biol. Chem. 277:38915–38920 (2002).
M. C. Zatelli, A. Luchin, D. Piccin, F. Tagliati, A. Bottoni, C. Vignali, M. Bondanelli, and E. C. degli Uberti. Cyclooxygenase-2 inhibitors reverse chemoresistance phenotype in medullary thyroid carcinoma by a permeability glycoprotein-mediated mechanism. J. Clin. Endocrinol. Metab. 90:5754–5760 (2005).
A. S. Kalgutkar, A. B. Marnett, B. C. Crews, R. P. Remmel, and L. J. Marnett. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J. Med. Chem. 43:2860–2870 (2000).
T. Mosmann. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65:55–63 (1983).
M. Thamotharan, S. Z. Bawani, X. Zhou, and S. A. Adibi. Hormonal regulation of oligopeptide transporter pept-1 in a human intestinal cell line. Am. J. Physiol. 276:C821–C826 (1999).
S. Siissalo, L. Laitinen, M. Koljonen, K. S. Vellonen, H. Kortejarvi, A. Urtti, J. Hirvonen, and A. M. Kaukonen. Effect of cell differentiation and passage number on the expression of efflux proteins in wild type and vinblastine-induced Caco-2 cell lines. Eur. J. Pharm. Biopharm. 67:548–54 (2007).
A. Pfrunder, H. Gutmann, C. Beglinger, and J. Drewe. Gene expression of CYP3A4, ABC-transporters (MDR1 and MRP1-MRP5) and hPXR in three different human colon carcinoma cell lines. J. Pharm. Pharmacol. 55:59–66 (2003).
A. Geick, M. Eichelbaum, and O. Burk. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J. Biol. Chem. 276:14581–14587 (2001).
D. Ratnasinghe, P. J. Daschner, M. R. Anver, B. H. Kasprzak, P. R. Taylor, G. C. Yeh, and J. A. Tangrea. Cyclooxygenase-2, P-glycoprotein-170 and drug resistance; is chemoprevention against multidrug resistance possible? Anticancer Res. 21:2141–2147 (2001).
D. Kessel, W. T. Beck, D. Kukuruga, and V. Schulz. Characterization of multidrug resistance by fluorescent dyes. Cancer Res. 51:4665–4670 (1991).
M. Fontaine, W. F. Elmquist, and D. W. Miller. Use of rhodamine 123 to examine the functional activity of P-glycoprotein in primary cultured brain microvessel endothelial cell monolayers. Life Sci. 59:1521–1531 (1996).
Y. Honjo, C. A. Hrycyna, Q. W. Yan, W. Y. Medina-Perez, R. W. Robey, A. van de Laar, T. Litman, M. Dean, and S. E. Bates. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 61:6635–6639 (2001).
J. D. Allen, S. C. Jackson, and A. H. Schinkel. A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistance. Cancer Res. 62:2294–2299 (2002).
O. Alqawi, S. Bates, and E. Georges. Arginine482 to threonine mutation in the breast cancer resistance protein ABCG2 inhibits rhodamine 123 transport while increasing binding. Biochem. J. 382:711–716 (2004).
R. W. Robey, Y. Honjo, A. van de Laar, K. Miyake, J. T. Regis, T. Litman, and S. E. Bates. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim. Biophys. Acta. 1512:171–182 (2001).
K. E. Pedersen, A. Dorph-Pedersen, S. Hvidt, N. A. Klitgaard, and K. K. Pedersen. The long-term effect of verapamil on plasma digoxin concentration and renal digoxin clearance in healthy subjects. Eur. J. Clin. Pharmacol. 22:123–127 (1982).
U. Puhlmann, C. Ziemann, G. Ruedell, H. Vorwerk, D. Schaefer, C. Langebrake, P. Schuermann, U. Creutzig, and D. Reinhardt. Impact of the cyclooxygenase system on doxorubicin-induced functional multidrug resistance 1 overexpression and doxorubicin sensitivity in acute myeloid leukemic HL-60 cells. J. Pharmacol. Exp. Ther. 312:346–354 (2005).
I. Tegeder, J. Pfeilschifter, and G. Geisslinger. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 15:2057–2072 (2001).
M. Bentires-Alj, V. Barbu, M. Fillet, A. Chariot, B. Relic, N. Jacobs, J. Gielen, M. P. Merville, and V. Bours. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 22:90–97 (2003).
M. L. Smith, G. Hawcroft, and M. A. Hull. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur. J. Cancer. 36:664–74 (2000).
M. Goto, S. Masuda, H. Saito, and K. Inui. Decreased expression of P-glycoprotein during differentiation in the human intestinal cell line Caco-2. Biochem. Pharmacol. 66:163–170 (2003).
Acknowledgments
This study was supported in part by grants from the university of Tishrin, Lattakia, Syrian Arabic republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zrieki, A., Farinotti, R. & Buyse, M. Cyclooxygenase Inhibitors Down Regulate P-glycoprotein in Human Colorectal Caco-2 Cell Line. Pharm Res 25, 1991–2001 (2008). https://doi.org/10.1007/s11095-008-9596-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9596-1