Abstract
Purpose
At high relative humidity (RH), orthorhombic paracetamol (form II) crystallized from ethanol transforms to monoclinic (form I) faster than such crystallized from the melt. The present study attempts to elucidate the reasons for this difference in stability.
Methods
The transformation of form II was investigated by powder X-ray diffraction, optical microscopy, gravimetric moisture sorption, thermogravimetry, and vibrational spectroscopy.
Results
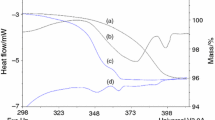
Solution-grown form II was found to be always contaminated with form I nuclei but still transforms much faster than corresponding physical mixtures of the pure forms in high RH, at a rate that is depending on the RH and the size of the crystals. A 0.1–0.6% w/w mass loss, inversely related to the initial monoclinic content, was observed during transformation of solution-grown form II, and was found to be due to residual ethanol that could not be removed by grinding, indicating incorporation by a solid solution mechanism.
Conclusions
Moisture triggers the growth of existing form I nuclei but it exerts a weaker effect on nucleation, and the presence of residual ethanol greatly accelerates the transformation.










Similar content being viewed by others
References
M. Haisa, S. Kashino, R. Kawai, and H. Maeda. The monoclinic form of p-hydroxyacetanilide. Acta Cryst. B32:1283–1285 (1976).
M. Haisa, S. Kashino, and H. Maeda. The orthorhombic form of p-hydroxyacetanilide. Acta Cryst. B30:2510–2512 (1974).
M. Peterson, Sh. Morissette, C. McNulty, A. Goldsweig, P. Shaw, M. LeQuesne, J. Monagle, N. Encina, J. Marchionna, A. Johnson, J. Gonzalez-Zugasti, A. Lemmo, S. Ellis, M. Cima, and Ö. Almarsson. Iterative high-throughput polymorphism studies on acetaminophen and an experimentally derived structure for form III. J. Am. Chem. Soc. 124:10958–10959 (2002).
I. Oswald, D. Allan, P. McGregor, S. Motherwell, S. Parsons, and C. Pulham. The formation of paracetamol (acetaminophen) adducts with hydrogen-bond acceptors. Acta Cryst. B58:1057–1066 (2002).
P. McGregor, D. Allan, S. Parsons, and C. Pulham. Preparation and crystal structure of a trihydrate of paracetamol. J. Pharm. Sci. 91:1308–1311 (2002).
A. Parkin, S. Parsons, and C. Pulham. Paracetamol monohydrate at 150 K. Acta Cryst. B58:o1345–o1347 (2002).
P. Di Martino, A. M. Guyot Hermann, P. Conflant, M. Drache, and J. C. Guyot. A new pure paracetamol for direct compression: the orthorhombic form. Int. J. Pharm. 128:1–8 (1996).
A. Burger, and R. Ramberger. On the polymorphism of pharmaceuticals and other molecular crystals. II. Mikrochim. Acta II:273–316 (1979).
L. Yu. Inferring thermodynamic stability relationship of polymorphs from melting data. J. Pharm. Sci. 84:966–974 (1995).
M. Sacchetti. Thermodynamic analysis of DSC data for acetaminophen polymorphs. J. Thermal Anal. 63:345–350 (2001).
E. Boldyreva, V. Drebushchak, I. Paukov, Y. Kovalevskaya, and T. Drebushchak. DSC and adiabatic calorimetry study of the polymorphs of paracetamol. An old problem revisited. J. Thermal. Anal. 77:607–623 (2004).
J. Ledru, C. Imrie, C. Pulham, R. Céolin, and J. Hutchinson. High pressure differential scanning calorimetry investigations on the pressure dependence of the melting of paracetamol polymorphs I and II. J. Pharm. Sci. 96:2784–2794 (2007).
G. Perlovich, T. Volkova, and A. Bauer-Brandl. Polymorphism of paracetamol. Relative stability of the monoclinic and orthorhombic phase revisited by sublimation and solution calorimetry. J. Thermal. Anal. 89:767–774 (2007).
G. Nichols, and C.S. Frampton. Physicochemical characterization of the orthorhombic polymorph of paracetamol crystallized from solution. J. Pharm. Sci. 87:684–693 (1998).
M. Lang, A. Grzesiak, and J. Matzger. The use of polymer heteronuclei for crystalline polymorph selection. J. Am. Chem. Soc. 124:14834–14835 (2002).
F. Fabbiani, D. Allan, W. David, S. Moggach, S. Parsons, and C. Pulham. High-pressure recrystallisation—a route to new polymorphs and solvates. Cryst. Eng. Comm. 6:504–511 (2004).
M. Mikhailenko. Growth of large single crystals of the orthorhombic paracetamol. J. Cryst. Growth. 265:616–618 (2004).
N. Al Zoubi, I. Nikolakakis, and S. Malamataris. Crystallisation conditions and formation of orthorhombic paracetamol from ethanolic solution. J. Pharm. Pharmacol. 54:325–333 (2002).
N. Al Zoubi, K. Kachrimanis, and S. Malamataris. Effects of harvesting and cooling on crystallisation and transformation of orthorhombic paracetamol in ethanolic solution. Eur. J. Pharm. Sci. 17:13–21 (2002).
N. Al Zoubi, and S. Malamataris. Effects of initial concentration and seeding procedure on crystallisation of orthorhombic paracetamol from ethanolic solution. Int. J. Pharm. 260:123–135 (2003).
K. Kachrimanis, D. Braun, and U. J. Griesser. Quantitative analysis of paracetamol polymorphs in powder mixtures by FT-Raman spectroscopy and PLS regression. J. Pharm. Biomed. Anal. 43:407–412 (2006).
A. Calka, and A. Radlinski. The local value of the Avrami exponent: a new approach to devitrification of glassy metallic ribbons. Mater. Sci. Eng. 97:241–246 (1988).
J. W. Christian. The theory of transformations in metals and alloys, Part I. 2Pergamon Press, Oxford, 1975.
W. Ostwald. Studien über die Bildung und Umwandlung fester Körper. Z. Phys. Chem. 22:289–330 (1897).
J. Bernstein, R. Davey, and J. O. Henck. Concomitant polymorphs. Angew. Chem. Int. Ed. 38:3440–3461 (1999).
G. Zhang, and D. Grant. Incorporation mechanism of guest molecules in crystals: solid solution or inclusion? Int. J. Pharm. 181:61–70 (1999).
G. Zhang, and D. Grant. Formation of liquid inclusions in adipic acid crystals during recrystallization from aqueous solutions. Cryst. Growth Des. 5:319–324 (2005).
F. Herbstein. On the mechanism of some first-order enantiotropic solid-state phase transitions: from Simon through Ubbelohde to Mnyukh. Acta Cryst. B62:341–383 (2006).
Y. Mnyukh. Polymorphic transitions in crystals: nucleation. J. Cryst. Growth 32:371–377 (1976).
Y. Mnyukh, N. Paflinova, N. Petropavlov, and N. Uchvatova. Polymorphic transitions in molecular crystals—III. Transitions exhibiting unusual behaviour. J. Phys. Chem. Solids 36:127–144 (1975).
ICH Q3C (R3) Impurities: Guideline for residual solvents. International Conference on Harmonization (2005).
C. Witschi, and E. Dolker. Residual solvent in pharmaceutical products: acceptable limits, influences in physicochemical properties, analytical methods and documented values. Eur. J. Pharm. Biopharm. 43:215–242 (1997).
A. Cruz Cabeza, G. Day, W. Motherwell, and W. Jones. Solvent inclusion in form II carbamazepine. Chem. Comm. 16:1600–1602 (2007).
F. Fabbiani, L. Byrne, J. McKinnon, and M. Spackman. Solvent inclusion in the structural voids of form II carbamazepine: single-crystal X-ray diffraction, NMR spectroscopy and Hirshfeld surface analysis. Cryst. Eng. Comm. 9:728–731 (2007).
U. J. Griesser. The importance of solvates. In R. Hilfiker (ed.), Polymorphism in the Pharmaceutical Industry, Wiley-VCH, Weinheim, Germany, 2006, pp. 211–234.
Acknowledgements
The authors thank Dr. M. Szelagiewicz of Solvias AG, for the assistance in the TG-FTIR measurements, Katharina Winkler for preliminary studies on the moisture induced phase transitions in a diploma thesis at the University of Innsbruck and Prof. Bill David (ISIS Facility, Rutherford Appleton Laboratory, Chilton, UK) for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kachrimanis, K., Fucke, K., Noisternig, M. et al. Effects of Moisture and Residual Solvent on the Phase Stability of Orthorhombic Paracetamol. Pharm Res 25, 1440–1449 (2008). https://doi.org/10.1007/s11095-007-9529-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9529-4