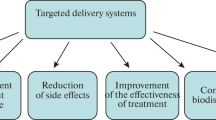
Abstract
Micelles, self-assembling nanosized colloidal particles with a hydrophobic core and hydrophilic shell are currently successfully used as pharmaceutical carriers for water-insoluble drugs and demonstrate a series of attractive properties as drug carriers. Among the micelle-forming compounds, amphiphilic copolymers, i.e., polymers consisting of hydrophobic block and hydrophilic block, are gaining an increasing attention. Polymeric micelles possess high stability both in vitro and in vivo and good biocompatibility, and can solubilize a broad variety of poorly soluble pharmaceuticals many of these drug-loaded micelles are currently at different stages of preclinical and clinical trials. Among polymeric micelles, a special group is formed by lipid-core micelles, i.e., micelles formed by conjugates of soluble copolymers with lipids (such as polyethylene glycol–phosphatidyl ethanolamine conjugate, PEG–PE). Polymeric micelles, including lipid-core micelles, carrying various reporter (contrast) groups may become the imaging agents of choice in different imaging modalities. All these micelles can also be used as targeted drug delivery systems. The targeting can be achieved via the enhanced permeability and retention (EPR) effect (into the areas with the compromised vasculature), by making micelles of stimuli-responsive amphiphilic block-copolymers, or by attaching specific targeting ligand molecules to the micelle surface. Immunomicelles prepared by coupling monoclonal antibody molecules to p-nitrophenylcarbonyl groups on the water-exposed termini of the micelle corona-forming blocks demonstrate high binding specificity and targetability. This review will discuss some recent trends in using micelles as pharmaceutical carriers.

Similar content being viewed by others
References
H. Müller. Colloidal Carriers for Controlled Drug Delivery and Targeting: Modification, Characterization, and In Vivo Distribution, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1991.
S. Cohen and H. Bernstein. Microparticulate Systems for the Delivery of Proteins and Vaccines, Marcel Dekker, New York, 1996.
D. D. Lasic and F. J. Martin. Stealth Liposomes, CRC, Boca Raton, Florida, 1995.
V. P. Torchilin and V. S. Trubetskoy. Which polymers can make nanoparticulate drug carriers long-circulating? Adv. Drug Deliv. Rev. 16:141–155 (1995).
T. N. Palmer, V. J. Caride, M. A. Caldecourt, J. Twickler, and V. Abdullah. The mechanism of liposome accumulation in infarction. Biochim. Biophys. Acta 797:363–368 (1984).
H. Maeda, J. Wu, T. Sawa, Y. Matsumura, and K. Hori. Tumor vascular permeability and the epr effect in macromolecular therapeutics: a review. J. Control. Release 65:271–284 (2000).
V. P. Torchilin. Polymer-coated long-circulating microparticulate pharmaceuticals. J. Microencapsul. 15:1–19 (1998).
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3–36 (2000).
B. A. Teicher. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval, Humana, Totowa, New Jersey, 1997.
A. M. Fernandez, K. Van Derpoorten, L. Dasnois, K. Lebtahi, V. Dubois, T. J. Lobl, S. Gangwar, C. Oliyai, E. R. Lewis, D. Shochat, and A. Trouet. N-succinyl-(beta-alanyl-l-leucyl-l-alanyl-l-leucyl)doxorubicin: an extracellularly tumor-activated prodrug devoid of intravenous acute toxicity. J. Med. Chem. 44:3750–3753 (2001).
S. H. Yalkowsky. Techniques of Solubilization of Drugs, Marcel Dekker, New York, 1981.
C. A. Lipinski. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 44:235–249 (2000).
B. A. Shabner and G. M. Collings (eds.), Cancer Chemotherapy: Principles and Practice, J.B. Lippincott, Philadelphia, 1990.
K. Yokogawa, E. Nakashima, J. Ishizaki, H. Maeda, T. Nagano, and F. Ichimura. Relationships in the structure-tissue distribution of basic drugs in the rabbit. Pharm. Res. 7:691–696 (1990).
A. Hageluken, L. Grunbaum, B. Nurnberg, R. Harhammer, W. Schunack, and R. Seifert. Lipophilic beta-adrenoceptor antagonists and local anesthetics are effective direct activators of g-proteins. Biochem. Pharmacol. 47:1789–1795 (1994).
D. Thompson and M. V. Chaubal. Cyclodextrins (CDS)—excipients by definition, drug delivery systems by function (part I: injectable applications). Drug Deliv. Technol. 2:34–38 (2000).
H. C. Ansel, L. V. Allen, and N. G. Popovich. Pharmaceutical Dosage Forms and Drug Delivery Systems, Kluwer, Norwell, Massachusetts, 1999.
D. D. Lasic and D. Papahadjopoulos. Medical Applications of Liposomes, Elsevier, New York, 1998.
P. P. Constantinides, K. J. Lambert, A. K. Tustian, B. Schneider, S. Lalji, W. Ma, B. Wentzel, D. Kessler, D. Worah, and S. C. Quay. Formulation development and antitumor activity of a filter-sterilizable emulsion of paclitaxel. Pharm. Res. 17:175–182 (2000).
R. Ray, A. H. Kibbe, R. Rowe, P. Shleskey, and P. Weller. Handbook of Pharmaceutical Excipients, APhA, Washington, District of Columbia, 2003.
M. J. Rosen (ed.), Surfactants and Interfacial Phenomena, Wiley, New York, 1989.
K. L. Mittal and B. Lindman (eds.), Surfactants in Solution (vols. 1–3), Plenum, New York, 1991.
M. Jones and J. Leroux. Polymeric micelles—a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 48:101–111 (1999).
A. Martin (ed.), Physical Pharmacy. Lippinkott, Williams and Wilkins, Philadelphia, 1993.
D. D. Lasic. Mixed micelles in drug delivery. Nature 355: 279–280 (1992).
P. H. Elworthy, A. T. Florence, and C. B. Macfarlane (eds.), Solubilization by Surface Active Agents, Chapman & Hall, London, UK, 1968.
D. Attwood and A. T. Florence (eds.), Surfactant Systems, Chapman & Hall, London, UK, 1983.
A. V. Kabanov, E. V. Batrakova, and N. S. Melik-Nubarov et al. A new class of drug carriers; micells poly(oxyethylene)–poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood to brain. J. Control. Release 22:141–158 (1992).
G. S. Kwon. Diblock copolymer nanoparticles for drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 15:481–512 (1998).
M. Jones and J. Leroux. Polymeric micelles—a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 48:101–111 (1999).
V. P. Torchilin. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 73:137–172 (2001).
A. A. Gabizon. Liposome circulation time and tumor targeting: implications for cancer chemotherapy. Adv. Drug Deliv. Rev. 16:285–294 (1995).
G. S. Kwon and K. Kataoka. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 16:295–309 (1995).
R. K. Jain. Transport of molecules, particles, and cells in solid tumors. Ann. Rev. Biomed. Eng. 1:241–263 (1999).
F. Yuan, M. Dellian, M. Fukumura, M. Leunig, D. A. Berk, V. P. Torchilin, and R. K. Jain. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 55:3752–3756 (1995).
V. Weissig, K. R. Whiteman, and V. P. Torchilin. Accumulation of protein-loaded long-circulating micelles and liposomes in subcutaneous Lewis lung carcinoma in mice. Pharm. Res. 15:1552–1556 (1998).
V. S. Trubetskoy and V. P. Torchilin. Use of polyoxyethylene–lipid conjugates as long-circulating carriers for delivery of therapeutic and diagnostic agents. Adv. Drug Deliv. Rev. 16:311–320 (1995).
O. Soga, C. F. van Nostrum, M. Fens, C. J. Rijcken, R. M. Schiffelers, G. Storm, and W. E. Hennink. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control. Release 103:341–353 (2005).
D. Le Garrec, S. Gori, L. Luo, D. Lessard, D. C. Smith, M. A. Yessine, M. Ranger, and J. C. Leroux. Poly(N-vinylpyrrolidone)-block-poly(d,l-lactide) as a new polymeric solubilizer hydrophobic anticancer drugs: in vitro and in vivo evaluation. J. Control. Release 99:83–101 (2004).
X. Shuai, T. Merdan, A. K. Schaper, F. Xi, and T. Kissel. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug. Chem. 15:441–448 (2004).
F. Mathot, L. van Beijsterveldt, V. Preat, M. Brewster, and A. Arien. Intestinal uptake and biodistribution of novel polymeric micelles after oral administration. J. Control. Release 111:47–55 (2006).
E. K. Park, S. Y. Kim, S. B. Lee, and Y. M. Lee. Folate-conjugated methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric micelles for tumor-targeted drug delivery. J. Control. Release 109:158–168 (2005).
H. Gao, Y. W. Yang, Y. G. Fan, and J. B. Ma. Conjugates of poly(dl-lactic acid) with ethylenediamino or diethylenetriamino bridged bis(beta-cyclodextrin)s and their nanoparticles as protein delivery systems. J. Control. Release 112:301–311 (2006).
D. J. Pillion, J. A. Amsden, C. R. Kensil, and J. Recchia. Structure–function relationship among quillaja saponins serving as excipients for nasal and ocular delivery of insulin. J. Pharm. Sci. 85:518–524 (1996).
F. Lallemand, O. Felt-Baeyens, K. Besseghir, F. Behar-Cohen, and R. Gurny. Cyclosporine a delivery to the eye: a pharmaceutical challenge. Eur. J. Pharm. Biopharm. 56:307–318 (2003).
J. Liaw, S. F. Chang, and F. C. Hsiao. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (peo–ppo–peo) polymeric micelles. Gene Ther. 8:999–1004 (2001).
G. S. Kwon and K. Kataoka. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 16:295–309 (1995).
G. Gaucher, M. H. Dufresne, V. P. Sant, N. Kang, D. Maysinger, and J. C. Leroux. Block copolymer micelles: preparation, characterization and application in drug delivery. J. Control. Release 109:169–188 (2005).
H. M. Aliabadi and A. Lavasanifar. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 3:139–162 (2006).
L. Zhang and A. Eisenberg. Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science 268:1728–1731 (1995).
G. S. Kwon and T. Okano. Soluble self-assembled block copolymers for drug delivery. Pharm. Res. 16:597–600 (1999).
A. V. Kabanov, E. V. Batrakova, and V. Y Alakhov. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 82:189–212 (2002).
S. B. La, T. Okano, and K. Kataoka. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(beta-benzyl L-aspartate) block copolymer micelles. J. Pharm. Sci. 85:85–90 (1996).
R. Gref, A. Domb, P. Quellec, T. Blunk, R. H. Muller, J. M. Verbavatz, and R. Langer. The controlled intravenous delivery of drugs using peg-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 16:215–233 (1995).
S. A. Hagan, A. G. A. Coombes, and M. C. Garnett, et al. Polylactide-poly(ethelene glycol) copolymers as drug delivery systems. 1. Characterization of water dispersible micelle-forming systems. Langmuir 12:2153–2161 (1996).
T. Inoue, G. Chen, K. Nakamae, and A. S. Hoffman. An AB block copolymers of oligo(methyl methacrylate) and poly(acrylic acid) for micellar delivery of hydrophobic drugs. J. Control. Release 51:221–229 (1998).
R. J. Hunter. In Foundations of Colloid Science, Vol. 1, Oxford University Press, New York, 1991.
Z. Gao and A. A. Eisenberg. A model of micellization for block copolymers in solutions. Macromolecules 26:7353–7360 (1993).
C. M. Marques. Bunchy Micelles. Langmuir 13:1430–1433 (1997).
G. S. Kwon. Polymeric micelles for delivery of poorly water-soluble compounds. Crit. Rev. Ther. Drug Carr. Syst. 20: 357–403 (2003).
H. Otsuka, Y. Nagasaki, and K. Kataoka. PEGylated nanoparticles for biological and harmaceutical applications. Adv. Drug Deliv. Rev. 55:403–419 (2003).
M. L. Adams, A. Lavasanifar, and G. S. Kwon. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 92: 1343–1355 (2003).
A. N. Lukyanov and V. P. Torchilin. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv. Drug Deliv. Rev. 56:1273–1289 (2004).
A. V. Kabanov, P. Lemieux, S. Vinogradov, and V. Alakhov. Pluronic block copolymers: novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 54:223–233 (2002).
Y. Kakizawa and K. Kataoka. Block copolymer micelles for delivery of gene and related compounds. Adv. Drug Deliv. Rev. 54:203–222 (2002).
T. Morcol, P. Nagappan, L. Nerenbaum, A. Mitchell, and S. J. Bell. Calcium phosphate–PEG–insulin–casein (CAPIC) particles as oral delivery systems for insulin. Int. J. Pharm. 277: 91–97 (2004).
A. Abuchowski, T. van Es, N. C. Palczuk, J. R. McCoy, and F. F. Davis. Treatment of l5178y tumor-bearing bdf1 mice with a nonimmunogenic l-glutaminase-l-asparaginase. Cancer Treat. Rep. 63:1127–1132 (1979).
J. M. Harris, N. E. Martin, and M. Modi. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 40:539–551 (2001).
M. J. Roberts, M. D. Bentley, and J. M. Harris. Chemistry for peptide and protein pegylation. Adv. Drug Deliv. Rev. 54: 459–476 (2002).
F. M. Veronese and J. M. Harris. Introduction and overview of peptide and protein pegylation. Adv. Drug Deliv. Rev. 54: 453–456 (2002).
A. L. Klibanov, K. Maruyama, V. P. Torchilin, and L. Huang. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 268:235–237 (1990).
P. Calvo, B. Gouritin, I. Brigger, C. Lasmezas, J. Deslys, A. Williams, J. P. Andreux, D. Dormont, and P. Couvreur. Pegylated polycyanoacrylate nanoparticles as vector for drug delivery in prion diseases. J. Neurosci. Methods 111:151–155 (2001).
S. M. Moghimi. Chemical camouflage of nanospheres with a poorly reactive surface: towards development of stealth and target-specific nanocarriers. Biochim. Biophys. Acta 1590: 131–139 (2002).
R. Smith and C. Tanford. The critical micelle concentration of dipalmitoylphosphatidylcholine in water and water–methanol solutions. J. Mol. Biol. 67:75–83 (1972).
V. P. Torchilin, V. S. Trubetskoy, K. R. Whiteman, P. Caliceti, P. Ferruti, and F. M. Veronese. New synthetic amphiphilic polymers for steric protection of liposomes in vivo. J. Pharm. Sci. 84:1049–1053 (1995).
V. P. Torchilin, T. S. Levchenko, K. R. Whiteman, A. A. Yaroslavov, A. M. Tsatsakis, A. K. Rizos, E. V. Michailova, and M. I. Shtilman. Amphiphilic poly-n-vinylpyrrolidones: synthesis, properties and liposome surface modification. Biomaterials 22:3035–3044 (2001).
D. Le Garrec, J. Taillefer, J. E. Van Lier, V. Lenaerts, and J. C. Leroux. Optimizing pH-responsive polymeric micelles for drug delivery in a cancer photodynamic therapy model. J. Drug Target. 10:429–437 (2002).
S. D. Johnson, J. M. Anderson, and R. E. Marchant. Biocompatibility studies on plasma polymerized interface materials encompassing both hydrophobic and hydrophilic surfaces. J. Biomed. Mater. Res. 26:915–35 (1992).
V. P. Torchilin, M. I. Shtilman, V. S. Trubetskoy, K. R. Whiteman, and A. M. Milstein. Amphiphilic vinyl polymers effectively prolong liposome circulation time in vivo. Biochim. Biophys. Acta 1195:181–184 (1994).
D. Sharma, T. P. Chelvi, J. Kaur, K. Chakravorty, T. K. De, A. Maitra, and R. Ralhan. Novel Taxol formulation: polyvinylpyrrolidone nanoparticle-encapsulated Taxol for drug delivery in cancer therapy. Oncol. Res. 8:281–286 (1996).
M. Moneghini, D. Voinovich, F. Princivalle, and L. Magarotto. Formulation and evaluation of vinylpyrrolidone/vinylacetate copolymer microspheres with carbamazepine. Pharm. Dev. Technol. 5:347–353 (2000).
A. Benahmed, M. Ranger, and J. C. Leroux. Novel polymeric micelles based on the amphiphilic diblock copolymer poly(N-vinyl-2-pyrrolidone)-block-poly(d,l-lactide). Pharm. Res. 18: 323–328 (2001).
B. Luppi, I. Orienti, F. Bigucci, T. Cerchiara, G. Zuccari, S. Fazzi, and V. Zecchi. Poly(vinylalcohol-co-vinyloleate) for the preparation of micelles enhancing retinyl palmitate transcutaneous permeation. Drug Deliv. 9:147–152 (2002).
B. Luppi, F. Bigucci, T. Cerchiara, V. Andrisano, V. Pucci, R. Mandrioli, and V. Zecchi. Micelles based on polyvinyl alcohol substituted with oleic acid for targeting of lipophilic drugs. Drug Deliv. 12:21–26 (2005).
Y. S. Nam, H. S. Kang, J. Y. Park, T. G. Park, S. H. Han, and I. S. Chang. New micelle-like polymer aggregates made from PEI-PLGA diblock copolymers: micellar characteristics and cellular uptake. Biomaterials 24:2053–2059 (2003).
A. V. Kabanov, V. P. Chekhonin, V. Alakhov, E. V. Batrakova, A. S. Lebedev, N. S. Melik-Nubarov, S. A. Arzhakov, A. V. Levashov, G. V. Morozov, and E. S. Severin et al. The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles. Micelles as microcontainers for drug targeting. FEBS Lett. 258:343–345 (1989).
D. W. Miller, E. V. Batrakova, T. O. Waltner, V. Yu. Alakhov, and A. V. Kabanov. Interactions of pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjug. Chem. 8: 649–657 (1997).
S. Katayose and K. Kataoka. Remarkable increase in nuclease resistance of plasmid DNA through supramolecular assembly with poly(ethylene glycol)-poly(L-lysine) block copolymer. J. Pharm. Sci. 87:160–163 (1998).
V. S. Trubetskoy, G. S. Gazelle, G. L. Wolf, and V. P. Torchilin. Block-copolymer of polyethylene glycol and polylysine as a carrier of organic iodine: design of long-circulating particulate contrast medium for x-ray computed tomography. J. Drug Target. 4:381–388 (1997).
M. Yokoyama, M. Miyauchi, N. Yamada, T. Okano, Y. Sakurai, K. Kataoka, and S. Inoue. Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res. 50:1693–1700 (1990).
A. Harada and K. Kataoka. Novel polyion complex micelles entrapping enzyme molecules in the core. Preparation of narrowly-distributed micelles from lysozyme and poly(ethylene glycol)-poly(aspartic acid) block copolymer in aqueous medium. Macromolecules 31:288–294 (1998).
G. S. Kwon, M. Naito, M. Yokoyama, T. Okano, Y. Sakurai, and K. Kataoka. Physical entrapment of adriamycin in AB block copolymer micelles. Pharm. Res. 12:192–195 (1995).
G. S. Kwon, M. Naito, M. Yokoyama, T. Okano, Y. Sakurai, and K. Kataoka. Block copolymer micelles for drug delivery: loading and release of doxorubicin. J. Control. Release 48: 195–201 (1997).
Y. I. Jeong, J. B. Cheon, S. H. Kim, J. W. Nah, Y. M. Lee, and Y. K. Sung et al. Clonazepam release from core-shell type nanoparticles in vitro. J. Control. Release 51:169–178 (1998).
S. Y. Kim, I. G. Shin, Y. M. Lee, C. G. Cho, and Y. K. Sung. Metoxy poly(ethylene glucol) and ɛ-caprolactone amphiphilic block copolymeric micelle containing indomethacin. II. Micelle formation and drug release behaviors. J. Control. Release 51:13–22 (1998).
C. Allen, Y. Yu, D. Maysinger, and A. Eisenberg. Polycaprolactone-b-poly(ethylene oxide) block copolymer micelles as a novel drug delivery vehicle for neurotrophic agents FK506 and L-685,818. Bioconjug. Chem. 9:564–572 (1998).
M. Ramaswamy, X. Zhang, H. Burt, and K. M. Wasan. Human plasma distribution of free paclitaxel and paclitaxel associated with diblock copolymers. J. Pharm. Sci. 86:460–464 (1997).
A. V. Kabanov and V. A. Kabanov. Interpolyelectrolyte and block ionomer complexes for gene delivery: physico-chemical aspects. Adv. Drug Deliv. Rev. 30:49–60 (1990).
V. Toncheva, E. Schacht, S. Y. Ng, J. Barr, and J. Heller. Use of block copolymers of poly(ortho esters) and poly (ethylene glycol) micellar carriers as potential tumour targeting systems. J. Drug Target. 11:345–353 (2003).
Y. Tang, S. Y. Liu, S. P. Armes, and N. C. Billingham. Solubilization and controlled release of a hydrophobic drug using novel micelle-forming ABC triblock copolymers. Biomacromolecules 4:1636–1645 (2003).
W. J. Lin, L. W. Juang, and C. C. Lin. Stability and release performance of a series of pegylated copolymeric micelles. Pharm. Res. 20:668–673 (2003).
S. C. Lee, C. Kim, I. Chan Kwon, H. Chung, and S. Young Jeong. Polymeric micelles of poly(2-ethyl-2-oxazoline)-block-poly(epsilon-caprolactone) copolymer as a carrier for paclitaxel. J. Control. Release 89:437–446. (2003).
G. B. Jiang, D. Quan, K. Liao, and H. Wang. Novel polymer micelles prepared from chitosan grafted hydrophobic palmitoyl groups for drug delivery. Mol. Pharmacol. 3:152–160 (2006).
S. Weiping, Y. Changqing, C. Yanjing, Z. Zhiguo, and K. Xiangzheng. Self-assembly of an amphiphilic derivative of chitosan and micellar solubilization of puerarin. Colloids Surf., B Biointerfaces 48:13–16 (2006).
A. V. Ambade, E. N. Savariar, and S. Thayumanavan. Dendrimeric micelles for controlled drug release and targeted delivery. Mol. Pharmacol. 2:264–272 (2005).
D. Bhadra, S. Bhadra, and N. K. Jain. Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J. Pharm. Pharm. Sci. 8:467–482 (2005).
K. Prompruk, T. Govender, S. Zhang, C. D. Xiong, and S. Stolnik. Synthesis of a novel peg-block-poly(aspartic acid-stat-phenylalanine) copolymer shows potential for formation of a micellar drug carrier. Int. J. Pharm. 297:242–253 (2005).
J. Djordjevic, M. Barch, and K. E. Uhrich. Polymeric micelles based on amphiphilic scorpion-like macromolecules: novel carriers for water-insoluble drugs. Pharm. Res. 22:24–32 (2005).
L. Tao, and K. E. Uhrich. Novel amphiphilic macromolecules and their in vitro characterization as stabilized micellar drug delivery systems. J. Colloid Interface Sci. 298:102–110 (2006).
F. Wang, T. K. Bronich, A. V. Kabanov, R. D. Rauh, and J. Roovers. Synthesis and evaluation of a star amphiphilic block copolymer from poly(epsilon-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconjug. Chem. 16:397–405 (2005).
H. Arimura, Y. Ohya, and T. Ouchi. Formation of core-shell type biodegradable polymeric micelles from amphiphilic poly(aspartic acid)-block-polylactide diblock copolymer. Biomacromolecules 6:720–725 (2005).
D. D. Lasic, M. C. Woodle, F. J. Martin, and T. Valentincic. Phase behavior of “stealth-lipid” decithin mixtures. Period. Biol. 93:287–290 (1991).
S. J. Duquemin and J. R. Nixon. The effect of sodium lauryl sulphate, cetrimide and polysorbate 20 surfactants on complex coacervate volume and droplet size. J. Pharm. Pharmacol. 37: 698–702 (1985).
V. T. Torchilin, A. N. Lukyanov, Z. Gao, and B. Papahadjopoulos-Sternberg. Proc. Natl. Acad. Sci. USA 100:6039–6044. (2003).
A. N. Lukyanov, Z. Gao, L. Mazzola, and V. P. Torchilin. Polyethylene glycol-diacyllipid micelles demonstrate increased acculumation in subcutaneous tumors in mice. Pharm. Res. 19: 1424–1429 (2002).
A. N. Lukyanov, W. C. Hartner, and V. P. Torchilin. Increased accumulation of peg-pe micelles in the area of experimental myocardial infarction in rabbits. J. Control. Release 94:187–193 (2004).
J. Wang, D. A. Mongayt, A. N. Lukyanov, T. S. Levchenko, and V. P. Torchilin. Preparation and in vitro synergistic anticancer effect of vitamin k3 and 1,8-diazabicyclo[5,4,0]undec-7-ene in poly(ethylene glycol)-diacyllipid micelles. Int. J. Pharm. 272:129–135 (2004).
A. Krishnadas, I. Rubinstein, and H. Onyuksel. Sterically stabilized phospholipid mixed micelles: in vitro evaluation as a novel carrier for water-insoluble drugs. Pharm. Res. 20:297–302 (2003).
Z. Gao, A. Lukyanov, A. Singhal, and V. Torchilin. Diacylipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2:979–982 (2002).
R. Nagarajan and K. Ganesh. Block copolymer self-assembly in selective solvents: theory of solubilization in spherical micelles. Macromolecules 22:4312–4325 (1989).
L. Xing, and W. L. Mattice. Large internal structures of micelles of triblock copolymers with small insoluble molecules in their cores. Langmuir 14:4074–4080 (1998).
C. Allen, D. Maysinger and A. Eisenberg. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf., B Biointerfaces 16:1–35 (1999).
S. Y. Lin and Y. Kawashima. The influence of three poly(oxyethylene)poly(oxypropylene) surface-active block copolymers on the solubility behavior of indomethacin. Pharm. Acta Helv. 60:339–344 (1985)
S. Y. Lin and Y. Kawashima. Pluronic surfactants affecting diazepam solubility, compatibility, and adsorption from i.v. admixture solutions. J. Parenter. Sci. Technol. 41:83–87 (1987).
M. Yokoyama, T. Okano, and K. Kataoka. Improved synthesis of adriamycin-conjugated poly(ethylene oxide)-poly(aspartic acid) block copolymer and formation of unimodal micellar structure with controlled amount of physically entrapped adriamycin. J. Control. Release 32:269–277 (1994).
M. Yokoyama, S. Fukushima, R. Uehara, K. Okamoto, K. Kataoka, and Y. Sakurai et al. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J. Control. Release 50:79–92 (1998).
M. Yokoyama, A. Satoh, Y. Sakurai, T. Okano, Y. Matsumura, and T. Kakizoe et al. Incorporation of water-insoluble anticancer drug into polymeric micelles and control of their particle size. J. Control. Release 55:219–229 (1998).
E. V. Batrakova, T. Y. Dorodnych, E. Y. Klinskii, E. N. Kliushnenkova, O. Shemchukova, and O. N. Goncharova et al. Anthracycline antibiotics non-covalently incorporated into the block copolymer micelles: in vivo evaluation of anti-cancer activity. Brit. J. Cancer 74:1545–1552 (1996).
A. V. Kabanov, I. R. Nazarova, I. R. Astafieva, E. V. Batrakova V. Yu. Alakhov, and A. A. Yaroslavov et al. Micelle formation and solubilization of fluorescence probes in poly(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules 28:2303–2314 (1995).
A. V. Kabanov, S. V. Vinogradov, U. G. Suzdaltseva, and V. Yu. Alakhov. Water-soluble block polycations as carriers for oligonucleotide delivery. Bioconjug. Chem. 6:639–643 (1995).
V. Yu. Alakhov and A. V. Kabanov. Block copolymeric biotransport carriers as versatile vehicles for drug delivery. Expert Opin. Investig. Drugs 7:1453–1473 (1998).
Y. Matsumura, M. Yokoyama, K. Kataoka, T. Okano, Y. Sakurai and T. Kawaguchi et al. Reduction of the side effects of an antitumor agent, KRN5500, by incorporation of the drug into polymeric micelles. Jpn. J. Cancer Res. 90:122–128 (1999).
G. S. Kwon, M. Yokoyama, T. Okano, Y. Sakurai, and K. Kataoka. Biodistribution of micelle-forming polymer–drug conjugates. Pharm. Res. 10:970–974 (1993).
G. S. Kwon, S. Suwa, M. Yokoyama, T. Okano, Y. Sakurai, and K. Kataoka. Enhanced tumor accumulation and prolonged circulation times of micelles-forming poly(ethylene oxide-aspartate) block copolymers-adriamycin conjugates. J. Control. Release 29:17–23 (1994).
K. Kataoka, G. S. Kwon, M. Yokoyama, T. Okano, and Y. Sakurai. Block-copolymer micelles as vehicles for drug delivery. J. Control. Release 24:119–132 (1993).
G. Kwon, M. Naito, M. Yokoyama, T. Okano, Y. Sakurai, and K. Kataoka. Micelles based on ab block copolymers of poly(ethylene oxide) and poly(benzyl-aspartat). Langmuir 9:945–949 (1993).
G. S. Kwon and T. Okano. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 21:107–116 (1996).
C. Allen, J. Han, Y. Yu, D. Maysinger, and A. Eisenberg. Polycaprolactone-b-poly(ethylene oxide) copolymer micelles as a delivery vehicle for dihydrotestosterone. J. Control. Release 63:275–286 (2000).
V. S. Trubetskoy and V. P. Torchilin. Polyethylene glycol based micelles as carriers of therapeutic and diagnostic agents. STP Pharma. Sci. 6:79–86 (1996).
J. Wang, D. Mongayt, and V. P. Torchilin. Polymeric micelles for delivery of poorly soluble drugs: preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. J. Drug Target. 13:73–80 (2005).
K. M. Huh, S. C. Lee, Y. W. Cho, J. Lee, J. H. Jeong, and K. Park. Hydrotropic polymer micelle system for delivery of paclitaxel. J. Control. Release 101:59–68 (2005).
H. Lee, F. Zeng, M. Dunne, and C. Allen. Methoxy poly(ethylene glycol)-block-poly(delta-valerolactone) copolymer micelles for formulation of hydrophobic drugs. Biomacromolecules 6:3119–3128 (2005).
M. Watanabe, K. Kawano, M. Yokoyama, P. Opanasopit, T. Okano, and Y. Maitani. Preparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stability. Int. J. Pharm. 308:183–189 (2006).
L. Mu, T. A. Elbayoumi, and V. P. Torchilin. Mixed micelles made of poly(ethylene glycol)–phosphatidylethanolamine conjugate and d-alpha-tocopheryl polyethylene glycol 1000 succinate as pharmaceutical nanocarriers for camptothecin. Int. J. Pharm. 306:142–149 (2005).
P. Opanasopit, M. Yokoyama, M. Watanabe, K. Kawano, Y. Maitani, and T. Okano. Block copolymer design for camptothecin incorporation into polymeric micelles for passive tumor targeting. Pharm. Res. 21:2001–2008 (2004).
P. Xu, E. A. Van Kirk, S. Li, W. J. Murdoch, J. Ren, M. D. Hussain, M. Radosz, and Y. Shen. Highly stable core-surface-crosslinked nanoparticles as cisplatin carriers for cancer chemotherapy. Colloids Surf., B Biointerfaces 48:50–57 (2006).
A. A. Exner, T. M. Krupka, K. Scherrer, and J. M. Teets. Enhancement of carboplatin toxicity by pluronic block copolymers. J. Control. Release 106:188–197 (2005).
H. Cabral, N. Nishiyama, S. Okazaki, H. Koyama, and K. Kataoka. Preparation and biological properties of dichloro(1,2-diaminocyclohexane)platinum(ii) (dachpt)-loaded polymeric micelles. J. Control. Release 101:223–232 (2005).
H. M. Aliabadi, A. Mahmud, A. D. Sharifabadi, and A. Lavasanifar. Micelles of methoxy poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine a. J. Control. Release 104: 301–311 (2005).
H. M. Aliabadi, D. R. Brocks, and A. Lavasanifar. Polymeric micelles for the solubilization and delivery of cyclosporine a: pharmacokinetics and biodistribution. Biomaterials 26:7251–7259 (2005).
P. Lim Soo, J. Lovric, P. Davidson, D. Maysinger, and A. Eisenberg. Polycaprolactone-block-poly(ethylene oxide) micelles: a nanodelivery system for 17beta-estradiol. Mol. Pharmacol. 2:519–527 (2005).
R. Vakil and G. S. Kwon. Peg-phospholipid micelles for the delivery of amphotericin b. J. Control. Release 101:386–389 (2005).
J. Liu, F. Zeng, and C. Allen. Influence of serum protein on polycarbonate-based copolymer micelles as a delivery system for a hydrophobic anti-cancer agent. J. Control. Release 103: 481–497 (2005).
L. Ould-Ouali, M. Noppe, X. Langlois, B. Willems, P. Te Riele, P. Timmerman, M. E. Brewster, A. Arien, and V. Preat. Self-assembling peg-p(cl-co-tmc) copolymers for oral delivery of poorly water-soluble drugs: a case study with risperidone. J. Control. Release 102:657–668 (2005).
S. V. Vinogradov, E. V. Batrakova, S. Li, and A. V. Kabanov. Mixed polymer micelles of amphiphilic and cationic copolymers for delivery of antisense oligonucleotides. J. Drug Target. 12:517–526 (2004).
R. B. Greenwald, C. W. Gilbert, A. Pendri, C. D. Conover, J. Xia, and A. Martinez. Drug delivery systems: water soluble taxol 2′-poly(ethylene glycol) ester prodrugs-design and in vivo effectiveness. J. Med. Chem. 39:424–431 (1996).
M. Hans, K. Shimoni, D. Danino, S. J. Siegel, and A. Lowman. Synthesis and characterization of mpeg-pla prodrug micelles. Biomacromolecules 6:2708–2717 (2005).
Z. Gao, A. N. Lukyanov, A. R. Chakilam, and V. P. Torchilin. Peg-pe/phosphatidylcholine mixed immunomicelles specifically deliver encapsulated taxol to tumor cells of different origin and promote their efficient killing. J. Drug Target. 11:87–92 (2003).
S. Alkan-Onyuksel, H. B. Ramakrishnan, and J. M. Chai. Pezzuto, a mixed micellar formulation suitable for the parenteral administration of taxol. Pharm. Res. 11:206–212 (1994).
H. Maeda, T. Sawa, and T. Konno. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug smancs. J. Control. Release 74:47–61 (2001).
V. Weissig, K. R. Whiteman, and V. P. Torchilin. Accumulation of protein-loaded long-circulating micelles and liposomes in subcutaneous Lewis lung carcinoma in mice. Pharm. Res. 15:1552–1556 (1998).
F. Yuan, M. Leunig, S. K. Huang, D. A. Berk, D. Papahadjopoulos, and R. K. Jain. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 54:3352–3356 (1994).
S. K. Hobbs, W. L. Monsky, F. Yuan, W. G. Roberts, L. Griffith, V. P. Torchilin, and R. K. Jain. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U. S. A. 95:4607–4612 (1998).
W. L. Monsky, D. Fukumura, T. Gohongi, M. Ancukiewcz, H. A. Weich, and V. P. Torchilin et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 59:4129–4135 (1999).
M. Yokoyama, T. Okano, Y. Sakurai, S. Fukushima, K. Okamoto, and K. Kataoka. Selective delivery of adriamycin to a solid tumor using a polymeric micelle carrier system. J. Drug Target. 7:171–186 (1999).
M. J. Parr, D. Masin, P. R. Cullis, and M. B. Bally. Accumulation of liposomal lipid and encapsulated doxorubicin in murine Lewis lung carcinoma: the lack of beneficial effects by coating liposomes with poly(ethylene glycol). J. Pharmacol. Exp. Ther. 280:1319–1327 (1997).
G. Helmlinger, F. Yuan, M. Dellian, and R. K. Jain. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 3:177–182 (1997).
I. F. Tannock and D. Rotin. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 49:4373–4384 (1989).
F. Kohori, K. Sakai, T. Aoyagi, M. Yokoyama, Y. Sakurai, and T. Okano. Preparation an characterization of thermally responsive block copolymer micelles comprising poly(N-isopropylacrylamide-b-DL-lactide). J. Control. Release 55:87–98 (1998).
O. Meyer, D. Papahadjopoulos, and J. C. Leroux. Copolymers of N-isopropylacrylamide can trigger pH sensitivity to stable liposomes. FEBS Lett. 41:61–64 (1998).
S. Cammas, K. Suzuki, C. Sone, Y. Sakurai, K. Kataoka, and T. Okano. Thermorespensive polymer nanoparticles with a core-shell micelle structure as site specific drug carriers. J. Control. Release 48:157–164 (1997).
V. P. Sant, D. Smith, and J. C. Leroux. Novel pH-sensitive supramolecular assemblies for oral delivery of poorly water soluble drugs: preparation and characterization. J. Control. Release 97:301–312 (2004).
H. S. Yoo, E. A. Lee, and T. G. Park. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J. Control. Release 82:17–27 (2002).
M. C. Jones, M. Ranger, and J. C. Leroux. pH-sensitive unimolecular polymeric micelles: synthesis of a novel drug carrier. Bioconjug. Chem. 14:774–781 (2003).
C. H. Wang, C. H. Wang, and G. H. Hsiue. Polymeric micelles with a ph-responsive structure as intracellular drug carriers. J. Control. Release 108:140–149 (2005).
G. H. Hsiue, C. H. Wang, C. L. Lo, C. H. Wang, J. P. Li, and J. L. Yang. Environmental-sensitive micelles based on poly(2-ethyl-2-oxazoline)-b-poly(l-lactide) diblock copolymer for application in drug delivery. Int. J. Pharm. 317:69–75 (2006).
C. Giacomelli, L. Le Men, R. Borsali, J. Lai-Kee-Him, A. Brisson, S. P. Armes, and A. L. Lewis. Phosphorylcholine-based ph-responsive diblock copolymer micelles as drug delivery vehicles: light scattering, electron microscopy, and fluorescence experiments. Biomacromolecules 7:817–828 (2006).
W. S. Shim, S. W. Kim, E. K. Choi, H. J. Park, J. S. Kim, and D. S. Lee. Novel pH sensitive block copolymer micelles for solvent free drug loading. Macromol. Biosci. 6:179–186 (2006).
M. Hruby, C. Konak, and K. Ulbrich. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J. Control. Release 103:137–148 (2005).
Y. Bae, N. Nishiyama, S. Fukushima, H. Koyama, M. Yasuhiro, and K. Kataoka. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 16:122–130 (2005).
C. F. Van Norstrum, D. Naradovic, J. Barends, M. J. Van Steenbergen, and W. E. Hennink. Nanoparticles and hydrogels with transient stability from thermosensitive block copolymers. Proceedings of 30th CRS Meeting, UK (2003) #163.
H. Yan and K. Tsujii. Potential application of poly(n-isopropylacrylamide) gel containing polymeric micelles to drug delivery systems. Colloids Surf., B Biointerfaces 46:142–146 (2005).
H. Wei, X. Z. Zhang, Y. Zhou, S. X. Cheng, and R. X. Zhuo. Self-assembled thermoresponsive micelles of poly(n-isopropylacrylamide-b-methyl methacrylate). Biomaterials 27:2028–2034 (2006).
S. Q. Liu, Y. W. Tong, and Y. Y. Yang. Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly(n-isopropylacrylamide-co-n,n-dimethylacrylamide)-b-poly(d,l-lactide-c o-glycolide) with varying compositions. Biomaterials 26:5064–5074 (2005).
J. E. Chung, M. Yokoyama, M. Yamato, T. Aoyagi, Y. Sakurai, and T. Okano. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate). J. Control. Release 62:115–127 (1999).
N. Rapoport, W. G. Pitt, H. Sun, and J. L. Nelson. Drug delivery in polymeric micelles: from in vitro to in vivo. J. Control. Release 91:85–95 (2003).
Z. G. Gao, H. D. Fain, and N. Rapoport. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J. Control. Release 102:203–222 (2005).
V. P. Torchilin. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 61:2549–2559 (2004).
S. Vinogradov, E. Batrakova, S. Li, and A. Kabanov. Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells. Bioconjug. Chem. 10:851–860 (1999).
V. P. Chekhonin, A. V. Kabanov, Y. A. Zhirkov, and G. V. Morozov. Fatty acid acylated Fab-fragments of antibodies to neurospecific proteins as carriers for neuroleptic targeted delivery in brain. FEBS Lett. 287:149–152 (1991).
Y. Nagasaki, K. Yasugi, Y. Yamamoto, A. Harada, and K. Kataoka. Sugar-installed block copolymer micelles: their preparation and specific interaction with lectin molecules. Biomacromolecules 2:1067–1070 (2001).
E. Jule, Y. Nagasaki, and K. Kataoka. Lactose-installed poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles exhibit fast-rate binding and high affinity toward a protein bed simulating a cell surface. A surface plasmon resonance study. Bioconjug. Chem. 14:177–186 (2003).
M. Ogris, S. Brunner, S. Schuller, R. Kircheis, and E. Wagner. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 6:595–605 (1999).
P. R. Dash, M. L. Read, K. D. Fisher, K. A. Howard, M. Wolfert, D. Oupicky, V. Subr, J. Strohalm, K. Ulbrich, and L. W. Seymour. Decreased binding to proteins and cells of polymeric gene delivery vectors surface modified with a multivalent hydrophilic polymer and retargeting through attachment of transferrin. J. Biol. Chem. 275:3793–802 (2000).
C. P. Leamon, D. Weigl, and R. W. Hendren. Folate copolymer-mediated transfection of cultured cells. Bioconjug. Chem. 10:947–957. (1999).
C. P. Leamon and P. S. Low. Folate-mediated targeting: from diagnostics to drug and gene delivery. Drug Discov. Today 6:44–51 (2001).
J. H. Jeong, S. H. Kim, S. W. Kim, and T. G. Park. In vivo tumor targeting of odn-peg-folic acid/pei polyelectrolyte complex micelles. J. Biomater. Sci., Polym. Ed. 16:1409–1419 (2005).
E. S. Lee, K. Na, and Y. H. Bae. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 91:103–113 (2003).
E. S. Lee, K. Na, and Y. H. Bae. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant mcf-7 tumor. J. Control. Release 103:405–418 (2005).
V. P. Torchilin, T. S. Levchenko, A. N. Lukyanov, B. A. Khaw, A. L. Klibanov, R. Rammohan, G. P. Samokhin, and K. R. Whiteman. p-Nitrophenylcarbonyl-PEG–PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim. Biophys. Acta 1511:397–411 (2001).
L. Z. Iakoubov and V. P. Torchilin. A novel class of antitumor antibodies: nucleosome-restricted antinuclear autoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol. Res. 9:439–446 (1997).
J. H. Felgner, R. Kumar, C. N. Sridhar, C. J. Wheeler, Y. J. Tsai, R. Border, P. Ramsey, M. Martin, and P. L. Felgner. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 269:2550–2561 (1994).
T. Ota, M. Maeda, and M. Tatsuka. Cationic liposomes with plasmid DNA influence cancer metastatic capability. Anticancer Res. 22:4049–4052 (2002).
S. Kaiser, and M. Toborek. Liposome-mediated high-efficiency transfection of human endothelial cells. J. Vasc. Res. 38: 133–143 (2001).
M. R. Almofti, H. Harashima, Y. Shinohara, A. Almofti, Y. Baba, and H. Kiwada. Cationic liposome-mediated gene delivery: Biophysical study and mechanism of internalization. Arch. Biochem. Biophys. 410:246–253 (2003).
I. M. Hafez, N. Maurer, and P. R. Cullis. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 8:1188–1196 (2001).
R. Ni, Y. Nishikawa, and B. I. Carr. Cell growth inhibition by a novel vitamin k is associated with induction of protein tyrosine phosphorylation. J. Biol. Chem. 273:9906–9911 (1998).
V. P. Torchilin. Peg-based micelles as carriers of contrast agents for different imaging modalities. Adv. Drug Deliv. Rev. 54: 235–252 (2002).
U. P. Schmiedl, J. A. Nelson, L. Teng, F. Starr, R. Malek, and R. J. Ho. Magnetic resonance imaging of the hepatobiliary system: intestinal absorption studies of manganese mesoporphyrin. Acad. Radiol. 2:994–1001 (1995).
C. W. Grant, S. Karlik, and E. Florio. A liposomal MRI contrast agent: phosphatidylethanolamine-dtpa. Magn. Reson. Med. 11:236–243 (1989).
G. W. Kabalka, E. Buonocore, K. Hubner, M. Davis, and L. Huang. Gadolinium-labeled liposomes containing paramagnetic amphipathic agents: targeted MRI contrast agents for the liver. Magn. Reson. Med. 8:89–95 (1988).
E. Unger, T. Fritz, G. Wu, D. Shen, B. Kulik, T. New, M. Crowell, and N. Wilke. Liposomal MR contrast agents. J. Liposome Res. 4:811–834 (1994).
V. S. Trubetskoy, M. D. Frank-Kamenetsky, K. R. Whiteman, G. L. Wolf, and V. P. Torchilin. Stable polymeric micelles: lymphangiographic contrast media for gamma scintigraphy and magnetic resonance imaging. Acad. Radiol. 3:232–238 (1996).
V. S. Trubetskoy, and V. P. Torchilin. New approaches in the chemical design of Gd-containing liposomes for use in magnetic resonance imaging of lymph nodes. J. Liposome Res. 4:961–980 (1994).
G. Wolf. Targeted delivery of imaging agents: an overview. In V. P. Torchilin (ed.), Handbook of Targeted Delivery of Imaging Agents. CRC, Boca Raton, Florida, 1995, pp. 3–22.
W. Krause, J. Leike, A. Sachse, and G. Schuhmann-Giampieri. Characterization of iopromide liposomes. Invest. Radiol. 28: 1028–1032 (1993).
P. Leander. A new liposomal contrast medium for CT of the liver. An imaging study in a rabbit tumour model. Acta Radiol. 37:63–68 (1996).
V. P. Torchilin, M. D. Frank-Kamenetsky, and G. L. Wolf. CT visualization of blood pool in rats by using long-circulating, iodine-containing micelles. Acad. Radiol. 6:61–65 (1999).
V. I. Slepnev, L. E. Kuznetsova, A. N. Gubin, E. V. Batrakova, V. Alakhov, and A. V. Kabanov. Micelles of poly(oxyethylene)-poly(oxypropylene) block copolymer (pluronic) as a tool for low-molecular compound delivery into a cell: phosphorylation of intracellular proteins with micelle incorporated [gamma-32p]atp. Biochem. Int. 26:587–595 (1992).
T. Nakanishi, S. Fukushima, K. Okamoto, M. Suzuki, Y. Matsumura, M. Yokoyama, T. Okano, Y. Sakurai, and K. Kataoka. Development of the polymer micelle carrier system for doxorubicin. J. Control. Release 74:295–302 (2001).
H. Uchino, Y. Matsumura, T. Negishi, F. Koizumi, T. Hayashi, T. Honda, N. Nishiyama, K. Kataoka, S. Naito, and T. Kakizoe. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br. J. Cancer 93:678–687 (2005).
T. Y. Kim, D. W. Kim, J. Y. Chung, S. G. Shin, S. C. Kim, D. S. Heo, N. K. Kim, and Y. J. Bang. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 10:3708–3716 (2004).
T. Trimaille, K. Mondon, R. Gurny, and M. Moller. Novel polymeric micelles for hydrophobic drug delivery based on biodegradable poly(hexyl-substituted lactides). Int. J. Pharm. 319:147–154 (2006).
M. N. Sibata, A. C. Tedesco, and J. M. Marchetti. Photophysicals and photochemicals studies of zinc(ii) phthalocyanine in long time circulation micelles for photodynamic therapy use. Eur. J. Pharm. Sci. 23:131–138 (2004).
A. K. Gupta, S. Madan, D. K. Majumdar, and A. Maitra. Ketorolac entrapped in polymeric micelles: preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 209:1–14 (2000).
M. H. Dufresne, D. L. Garrec, V. Sant, J. C. Leroux, and M. Ranger. Preparation and characterization of water-soluble ph-sensitive nanocarriers for drug delivery. Int. J. Pharm. 277: 81–90 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm Res 24, 1–16 (2007). https://doi.org/10.1007/s11095-006-9132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9132-0