Abstract
Purpose
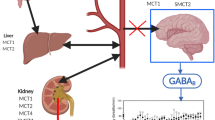
To investigate if γ-Hydroxybutyrate (GHB) tolerance is mediated by alterations in GHB systemic pharmacokinetics, transport (blood brain barrier (BBB) and neuronal) or membrane fluidity.
Materials and Methods
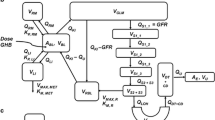
GHB tolerance in rats was attained by repeated GHB administration (5.31 mmol/kg, s.c., QD for 5 days). GHB sedative/hypnotic effects were measured daily. GHB pharmacokinetics were determined on day 5. In separate groups, on day 6, in situ brain perfusion was performed to assess BBB transport alterations; or in vitro studies were performed (fluorescence polarization measurements of neuronal membrane fluidity or [3H]GABA neuronal accumulation).
Results
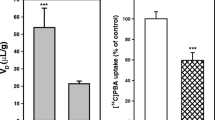
GHB sedative/hypnotic tolerance was observed by day 5. No significant GHB pharmacokinetic or BBB transport differences were observed between treated and control rats. Neuronal membrane preparations from GHB tolerant rats showed a significant decrease in fluorescence polarization (treated—0.320 ± 0.009, n = 5; control—0.299 ± 0.009, n = 5; p < 0.05). [3H]GABA neuronal transport V max was significantly increased in tolerant rats (2,110.66 ± 91.06 pmol/mg protein/min vs control (1,612.68 ± 176.03 pmol/mg protein/min; n = 7 p < 0.05).
Conclusions
Short term GHB administration at moderate doses results in the development of tolerance which is not due to altered systemic pharmacokinetics or altered BBB transport, but might be due to enhanced membrane rigidity and increased GABA reuptake.





Similar content being viewed by others
Abbreviations
- AUC:
-
area under curve
- AUMC:
-
area under first moment curve
- BBB:
-
blood brain barrier
- C :
-
perfusion fluid concentration of tracer
- CL/F :
-
systemic clearance
- CLin :
-
influx clearance
- C max :
-
maximum concentration
- CNS:
-
central nervous system
- DPH:
-
1,6-diphenyl-1,3,5-hexatriene
- GABA:
-
γ-aminobutyric acid
- GBL:
-
γ-butyrolactone
- GHB:
-
γ-hydroxybutyrate
- LRR:
-
loss in righting reflex
- MCT:
-
monocarboxylate acid transporter
- MRT:
-
mean residence time
- P :
-
fluorescence polarization
- Q :
-
mass of radiotracer in the brain region normalized for wet brain tissue weight
- r :
-
anisotropy
- RRR:
-
return in righting reflex
- S 2 :
-
lipid order
- T :
-
time of perfusion
- T 1/2 :
-
half life
- T max :
-
time to maximum concentration
- V/F:
-
volume of distribution
- V vasc :
-
regional volume of the cerebrovascular capillary bed
References
F. Caputo, G. Addolorato, F. Lorenzini, M. Domenicali, G. Greco, A. del RE, G. Gasbarrini, G. F. Stefanini, and M. Bernardi. Gamma-hydroxybutyric acid versus naltrexone in maintaining alcohol abstinence: an open randomized comparative study. Drug Alcohol Depend. 70:85–91 (2003).
P. Follesa, F. Biggio, L. Mancuso, S. Cabras, S. Caria, G. Gorini, A. Manca, A. Orru, and G. Biggio. Ethanol withdrawal-induced up-regulation of the alpha2 subunit of the GABAA receptor and its prevention by diazepam or gamma-hydroxybutyric acid. Mol. Brain Res. 120:130–137 (2004).
M. B. Scharf, M. Baumann, and D. V. Berkowitz. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J. Rheumatol. 30:1070–1074 (2003).
L. Gallimberti, M. Spella, C. Soncini, and G. Gessa. Gamma-hydroxybutyric acid (GHB) in treatment of alcohol and heroin dependence. Alcohol 20:257–262 (2000).
C. G. Wong, K. M. Gibson, and O. C. Snead, 3rd. From the street to the brain: neurobiology of the recreational drug gamma-hydroxybutyric acid. Trends Pharmacol. Sci. 25:29–34 (2004).
M. Mamelak. Neurodegeneration, sleep, and cerebral energy metabolism: a testable hypothesis. J. Geriatr. Psychiatry Neurol. 10:29–32 (1997).
G. Tunnicliff. Significance of gamma-hydroxybutyric acid in the brain. Gen. Pharmacol. 23:1027–1034 (1992).
J. E. Dyer. gamma-Hydroxybutyrate: a health-food product producing coma and seizurelike activity. Am. J. Emerg. Med. 9:321–324 (1991).
G. P. Galloway, S. L. Frederick, F. E. Staggers, Jr., M. Gonzales, S. A. Stalcup, and D. E. Smith. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence.[see comment]. Addiction 92:89–96 (1997).
G. Addolorato, E. Castelli, G. F. Stefanini, G. Casella, F. Caputo, L. Marsigli, M. Bernardi, and G. Gasbarrini. An open multicentric study evaluating 4-hydroxybutyric acid sodium salt in the medium-term treatment of 179 alcohol dependent subjects. GHB Study Group. Alcohol 31:341–345 (1996).
M. Mamelak, M. B. Scharf, and M. Woods. Treatment of narcolepsy with gamma-hydroxybutyrate. A review of clinical and sleep laboratory findings. Sleep 9:285–289 (1986).
M. B. Scharf, D. Brown, M. Woods, L. Brown, and J. Hirschowitz. The effects and effectiveness of gamma-hydroxybutyrate in patients with narcolepsy. J. Clin. Psychiatry 46:222–225 (1985).
T. C. Bania, T. Ashar, G. Press, and P. M. Carey. Gamma-hydroxybutyric acid tolerance and withdrawal in a rat model. Acad. Emerg. Med. 10:697–704 (2003).
Y. Itzhak and S. F. Ali. Repeated administration of gamma-hydroxybutyric acid (GHB) to mice: assessment of the sedative and rewarding effects of GHB. Ann. N. Y. Acad. Sci. 965:451–460 (2002).
D. K. Van Sassenbroeck, P. De Paepe, F. M. Belpaire, P. A. Boon, and W. A. Buylaert. Tolerance to the hypnotic and electroencephalographic effect of gamma-hydroxybutyrate in the rat: pharmacokinetic and pharmacodynamic aspects. J. Pharm. Pharmacol. 55:609–615 (2003).
O. Giorgi and M. C. Rubio. Decreased 3H-L-quinuclidinyl benzilate binding and muscarine receptor subsensitivity after chronic gamma-butyrolactone treatment. Naunyn Schmiedeberg's Arch. Pharmacol. 318:14–18 (1981).
C. Ratomponirina, S. Gobaille, Y. Hode, V. Kemmel, and M. Maitre. Sulpiride, but not haloperidol, up-regulates gamma-hydroxybutyrate receptors in vivo and in cultured cells. Eur. J. Pharmacol. 346:331–337 (1998).
H. Tsuchiya. Structure-specific membrane-fluidizing effect of propofol. Clin. Exp. Pharmacol. Physiol. 28:292–299 (2001).
S. V. Balasubramanian, R. B. Campbell, and R. M. Straubinger. Propofol, a general anesthetic, promotes the formation of fluid phase domains in model membranes. Chem. Phys. Lipids 114:35–44 (2002).
K. L. Kopnisky and S. E. Hyman. Molecular and cellular biology of addiction. In K. L. Davis and American College of Neuropsychopharmacology (eds.), Neuropsychopharmacology: The Fifth Generation of Progress: An Official Publication of the American College of Neuropsychopharmacology, Lippincott/Williams & Wilkins, Philadelphia, 2002, pp. xxi, 2010 p., [24] p. of plates.
M. Shinitzky. Physiology of Membrane Fluidity. CRC, Boca Raton, Florida, 1984.
H. J. Lee, S. V. Balasubramanian, H. Murer, J. Biber, and M. E. Morris. Modulation of sulfate renal transport by alterations in cell membrane fluidity. J. Pharm. Sci. 88:976–980 (1999).
S. V. Balasubramanian, R. M. Straubinger, and M. E. Morris. Salicylic acid induces changes in the physical properties of model and native kidney membranes. J. Pharm. Sci. 86:199–204 (1997).
S. Gobaille, V. Hechler, C. Andriamampandry, V. Kemmel, and M. Maitre. gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo. J. Pharmacol. Exp. Ther. 290:303–309 (1999).
V. Hechler, C. Ratomponirina, and M. Maitre. gamma-Hydroxybutyrate conversion into GABA induces displacement of GABAB binding that is blocked by valproate and ethosuximide. J. Pharmacol. Exp. Ther. 281:753–760 (1997).
E. M. Bernstein and M. W. Quick. Regulation of gamma-aminobutyric acid (GABA) transporters by extracellular GABA. J. Biol. Chem. 274:889–895 (1999).
I. Bhattacharya and K. M. Boje. GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood–brain barrier. J. Pharmacol. Exp. Ther. 311:92–98 (2004).
H. L. Fung, E. Haas, J. Raybon, J. Xu, and S. M. Fung. Liquid chromatographic–mass spectrometric determination of endogenous gamma-hydroxybutyrate concentrations in rat brain regions and plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 807:287–291 (2004).
V. P. Whittaker, I. A. Michaelson, and R. J. Kirkland. The separation of synaptic vesicles from nerve-ending particles (‘synaptosomes’). Biochem. J. 90:293–303 (1964).
G. R. Bartlett. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466–468 (1959).
O. Lowry, N. Rosebrough, A. Farr, and R. Randall. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275 (1951).
A. Pastuszko, D. F. Wilson, and M. Erecinska. Net uptake of gamma-aminobutyric acid by a high-affinity system of rat brain synaptosomes. Proc. Natl. Acad. Sci. U. S. A. 78:1242–1244 (1981).
J. H. Hu, Y. H. Ma, N. Yang, Z. T. Mei, M. H. Zhang, J. Fei, and L. H. Guo. Up-regulation of gamma-aminobutyric acid transporter I mediates ethanol sensitivity in mice. Neuroscience 123:807–812 (2004).
B. I. Kanner. Active transport of gamma-aminobutyric acid by membrane vesicles isolated from rat brain. Biochemistry 17:1207–1211 (1978).
J. T. Lettieri and H. L. Fung. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J. Pharmacol. Exp. Ther. 208:7–11 (1979).
W. van der Meer, H. Pottel, W. Herreman, M. Ameloot, H. Hendrickx, and H. Schroder. Effect of orientational order on the decay of the fluorescence anisotropy in membrane suspensions. A new approximate solution of the rotational diffusion equation. Biophys. J. 46:515–523 (1984).
E. Van Cauter, L. Plat, M. B. Scharf, R. Leproult, S. Cespedes, M. L'Hermite-Baleriaux, and G. Copinschi. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J. Clin. Invest. 100:745–753 (1997).
N. Moller, J. Gjedsted, L. Gormsen, J. Fuglsang, and C. Djurhuus. Effects of growth hormone on lipid metabolism in humans. Growth Horm. IGF Res. 13(Suppl A):S18–S21 (2003).
K. L. Nicholson and R. L. Balster. GHB: a new and novel drug of abuse. Drug Alcohol Depend. 63:1–22 (2001).
L. P. Carter, H. Wu, W. Chen, C. M. Cruz, R. J. Lamb, W. Koek, A. Coop, and C. P. France. Effects of gamma-hydroxybutyrate (GHB) on schedule-controlled responding in rats: role of GHB and GABAB receptors. J. Pharmacol. Exp. Ther. 308:182–188 (2004).
J. T. Lettieri and H. L. Fung. Improved pharmacological activity via pro-drug modification: comparative pharmacokinetics of sodium gamma-hydroxybutyrate and gamma-butyrolactone. Res. Commun. Chem. Pathol. Pharmacol. 22:107–118 (1978).
M. Maitre, V. Hechler, P. Vayer, S. Gobaille, C. D. Cash, M. Schmitt, and J. J. Bourguignon. A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties. J. Pharmacol. Exp. Ther. 255:657–663 (1990).
S. s. NCADI. GHB www.health.org/nongovpubs/ghbqa/, Vol. 2005.
A. Gjedde and C. Crone. Induction processes in blood–brain transfer of ketone bodies during starvation. Am. J. Physiol. 229:1165–1169 (1975).
M. Pollay and F. A. Stevens. Starvation-induced changes in transport of ketone bodies across the blood–brain barrier. J. Neurosci. Res. 5:163–172 (1980).
R. L. Leino, D. Z. Gerhart, R. Duelli, B. E. Enerson, and L. R. Drewes. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 38:519–527 (2001).
H. Stibler, F. Beauge, and S. Borg. Changes in (Na+ + K+) ATPase activity and the composition of surface carbohydrates in erythrocyte membranes in alcoholics. Alcohol. Clin. Exp. Res. 8:522–527 (1984).
D. L. Zvosec, S. W. Smith, J. R. McCutcheon, J. Spillane, B. J. Hall, and E. A. Peacock. Adverse events, including death, associated with the use of 1,4-butanediol. N. Engl. J. Med. 344:87–94 (2001).
M. A. Carai, G. Colombo, G. Brunetti, S. Melis, S. Serra, G. Vacca, S. Mastinu, A. M. Pistuddi, C. Solinas, G. Cignarella, G. Minardi, and G. L. Gessa. Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur. J. Pharmacol. 428:315–321 (2001).
M. L. Beckman, E. M. Bernstein, and M. W. Quick. Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A. J. Neurosci. 18:6103–6112 (1998).
M. L. Beckman, E. M. Bernstein, and M. W. Quick. Multiple G protein-coupled receptors initiate protein kinase C redistribution of GABA transporters in hippocampal neurons. J Neurosci. 19:RC9 (1999).
Acknowledgments
The authors would like to thank Mr. David Soda for his assistance with the jugular vein cannulations and Dr. S. Balasubramanian and his group for insightful discussions and suggestions for the membrane fluidity fluorescence studies. This work was supported in part by National Institutes of Health grant DA 14988 and a Merck predoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Indranil Bhattacharya and Joseph J. Raybon have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bhattacharya, I., Raybon, J.J. & Boje, K.M.K. Alterations in Neuronal Transport but not Blood-Brain Barrier Transport are Observed during Gamma-Hydroxybutyrate (GHB) Sedative/Hypnotic Tolerance. Pharm Res 23, 2067–2077 (2006). https://doi.org/10.1007/s11095-006-9066-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9066-6