No Heading
Purpose.
The objective was to assess the permeation and clearance of model ionic permeants after subconjunctival injection with nuclear magnetic resonance imaging (MRI).
Methods.
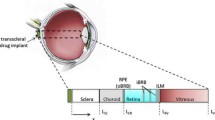
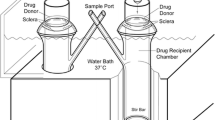
New Zealand white rabbit was the animal model and manganese ion (Mn2+) and manganese ethylenediaminetetraacetic acid complex (MnEDTA2−) were the model permeants. The current study was divided into three parts: in vitro, postmortem, and in vivo. Transscleral passive permeation experiments were conducted with excised sclera in side-by-side diffusion cells in vitro. Subconjunctival delivery experiments were conducted with rabbits postmortem and in vivo. The distribution and elimination of the probe permeants from the subconjunctival space after subconjunctival injections were determined by MRI.
Results.
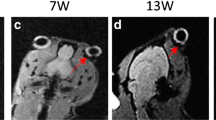
The data of excised sclera in vitro suggest large effective pore size for transscleral transport and negligible pore charge effects upon the permeation of the ionic permeants. The permeability coefficients of Mn2+ and MnEDTA2- across the sclera in vitro were 3.6 × 10-5 cm/s and 2.4 × 10-5 cm/s, respectively. Although relatively high sclera permeability was observed in vitro, subconjunctival injections in vivo did not provide significant penetration of Mn2+ and MnEDTA2- into the globe; permeant concentrations in the eye were below the detection limit, which corresponds to less than 0.05% of the concentration of the injection solution (e.g., less than 0.02 mM when 40 mM injection solution was used). The volume of the subconjunctival pocket and the concentration of the permeants in the pocket were observed to decrease with time after the injection, and this could contribute to the lower than expected subconjunctival absorption in vivo. Different from the results in vivo, experiments with rabbits postmortem show significant penetration of Mn2+ and MnEDTA2- into the globe with the permeants primarily delivered into the anterior segment of the eye. This difference suggests blood vasculature clearance as a main barrier for passive transscleral transport. The data also show that the pars plicata/pars plana is the least resistance pathway for passive transscleral drug delivery of the polar permeants, and there are indications of the presence of another barrier, possibly the retinal epithelium and/or Bruch’s membrane, at the back of the eye.
Conclusions.
Subconjunctival delivery of the ionic permeants in vivo cannot be quantitatively predicted by the in vitro results. MRI is a noninvasive complementary technique to traditional pharmacokinetic methods. It can provide insights into ocular pharmacokinetics without permeant redistribution that can occur in surgical procedure postmortem in traditional pharmacokinetic studies when the blood vasculature barrier is absent.
Similar content being viewed by others
References
1. T. W. Kim, J. D. Lindsey, M. Aihara, T. L. Anthony, and R. N. Weinreb. Intraocular distribution of 70-kDa dextran after subconjunctival injection in mice. Invest. Ophthalmol. Vis. Sci. 43:1809–1816 (2002).
2. Y. Yanagi, Y. Tamaki, R. Obata, K. Muranaka, N. Homma, H. Matsuoka, and H. Mano. Subconjunctival administration of bucillamine suppresses choroidal neovascularization in rat. Invest. Ophthalmol. Vis. Sci. 43:3495–3499 (2002).
3. U. B. Kompella, N. Bandi, and S. P. Ayalasomayajula. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest. Ophthalmol. Vis. Sci. 44:1192–1201 (2003).
4. N. Worakul and J. R. Robinson. Ocular pharmacokinetics/pharmacodynamics. Eur. J. Pharm. Biopharm. 44:71–83 (1997).
5. T. J. Zimmerman, K. S. Kooner, M. Sharir, and R. D. Fechtner. Textbook of Ocular Pharmacology, Lippincoot-Raven, Philadelphia, 1997.
6. D. H. Geroski and H. F. Edelhauser. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 52:37–48 (2001).
7. M. R. Prausnitz and J. S. Noonan. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci. 87:1479–1488 (1998).
8. J. Ambati, C. S. Canakis, J. W. Miller, E. S. Gragoudas, A. Edwards, D. J. Weissgold, I. Kim, F. C. Delori, and A. P. Adamis. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 41:1181–1185 (2000).
9. A. K. Mitra. Ophthalmic Drug Delivery Systems, Marcel Dekker, New York, 1993.
10. Y. Ogura. Drug delivery to the posterior segments of the eye. Adv. Drug Deliv. Rev. 52:1–3 (2001).
11. T. W. Lee and J. R. Robinson. Drug delivery to the posterior segment of the eye II: development and validation of a simple pharmacokinetic model for subconjunctival injection. J. Ocul. Pharmacol. Ther. 20:43–53 (2004).
12. T. W. Lee and J. R. Robinson. Drug delivery to the posterior segment of the eye III: the effect of parallel elimination pathway on the vitreous drug level after subconjunctival injection. J. Ocul. Pharmacol. Ther. 20:55–64 (2004).
13. S. K. Li, E. K. Jeong, and M. S. Hastings. Magnetic resonance imaging study of current and ion delivery into the eye during transscleral and transcorneal iontophoresis. Invest. Ophthalmol. Vis. Sci. 45:1224–1231 (2004).
14. D. D. Stark and W. G. Bradley. Magnetic Resonance Imaging, 2nd ed. Mosbey Year Book, Boston, 1992, Ch. 14.
15. T. Watanabe, T. Michaelis, and J. Frahm. Mapping of retinal projections in the living rat using high-resolution 3D gradient-echo MRI with Mn2+-induced contrast. Magn. Reson. Med. 46:424–429 (2001).
16. R. G. Pautler, R. Mongeau, and R. E. Jacobs. In vivo trans-synaptic tract tracing from the murine striatum and amygdala utilizing manganese enhanced MRI (MEMRI). Magn. Reson. Med. 50:33–39 (2003).
17. K. D. Peck, A. H. Ghanem, and W. I. Higuchi. Hindered diffusion of polar molecules through and effective pore radii estimates of intact and ethanol treated human epidermal membrane. Pharm. Res. 11:1306–1314 (1994).
18. D. R. Lide. CRC Handbook for Chemistry and Physics, CRC Press, New York, 2004.
19. S. K. Li, A. H. Ghanem, K. D. Peck, and W. I. Higuchi. Iontophoretic transport across a synthetic membrane and human epidermal membrane: a study of the effects of permeant charge. J. Pharm. Sci. 86:680–689 (1997).
20. D. E. Rudnick, J. S. Noonan, D. H. Geroski, M. R. Prausnitz, and H. F. Edelhauser. The effect of intraocular pressure on human and rabbit scleral permeability. Invest. Ophthalmol. Vis. Sci. 40:3054–3058 (1999).
21. M. E. Yablonski, M. Hayashi, D. J. Cook, G. Chubak, and M. Sirota. Fluorophotometric study of intravenous carbonic anhydrase inhibitors in rabbits. Invest. Ophthalmol. Vis. Sci. 28:2076–2082 (1987).
22. H. M. Cheng, K. K. Kwong, J. Xiong, and C. Chang. GdDTPA-enhanced magnetic resonance imaging of the aqueous flow in the rabbit eye. Magn. Reson. Med. 17:237–243 (1991).
23. M. Barza and M. McCue. Pharmacokinetics of aztreonam in rabbit eyes. Antimicrob. Agents Chemother. 24:468–473 (1983).
24. W. M. Jay, R. K. Shockley, A. M. Aziz, M. Z. Aziz, and J. P. Rissing. Ocular pharmacokinetics of ceftriaxone following subconjunctival injection in rabbits. Arch. Ophthalmol. 102:430–432 (1984).
25. S. Marrakchi-Benjaafar, I. Cochereau, F. D’Hermies, and J. J. Pocidalo. Tolerability, kinetics, and efficacy of subconjunctival pefloxacin in pigmented rabbits. Antimicrob. Agents Chemother. 39:834–838 (1995).
26. D. P. Hainsworth, J. D. Conklin, J. R. Bierly, D. Ax, and P. Ashton. Intravitreal delivery of ciprofloxacin. J. Ocul. Pharmacol. Ther. 12:183–191 (1996).
27. G. S. Kalsi, G. Gudauskas, N. Bussanich, D. J. Freeman, and J. Rootman. Ocular pharmacokinetics of subconjunctivally administered cyclosporine in the rabbit. Can. J. Ophthalmol. 26:200–205 (1991).
28. M. Barza, A. Kane, and J. L. Baum. Intraocular levels of cefamandole compared with cefazolin after subconjunctival injection in rabbits. Invest. Ophthalmol. Vis. Sci. 18:250–255 (1979).
29. E. F. Erkin, U. Gunenc, F. H. Oner, A. Gelal, Y. Erkin, and H. Guven. Penetration of amikacin into aqueous humor of rabbits. Ophthalmologica 215:299–302 (2001).
30. M. Souli, G. Kopsinis, E. Kavouklis, L. Gabriel, and H. Giamarellou. Vancomycin levels in human aqueous humour after intravenous and subconjunctival administration. Int. J. Antimicrob. Agents 18:239–243 (2001).
31. T. W. Lee and J. R. Robinson. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J. Ocul. Pharmacol. Ther. 17:565–572 (2001).
32. W. S. Foulds, D. Allan, H. Moseley, and P. M. Kyle. Effect of intravitreal hyaluronidase on the clearance of tritiated water from the vitreous to the choroid. Br. J. Ophthalmol. 69:529–532 (1985).
33. B. A. Berkowitz, C. A. Wilson, P. S. Tofts, and R. M. Peshock. Effect of vitreous fluidity on the measurement of blood-retinal barrier permeability using contrast-enhanced MRI. Magn. Reson. Med. 31:61–66 (1994).
34. T. L. Jackson, R. J. Antcliff, J. Hillenkamp, and J. Marshall. Human retinal molecular weight exclusion limit and estimate of species variation. Invest. Ophthalmol. Vis. Sci. 44:2141–2146 (2003).
35. B. A. Berkowitz, P. S. Tofts, H. A. Sen, N. Ando, and E. de Juan Jr. Accurate and precise measurement of blood-retinal barrier breakdown using dynamic Gd-DTPA MRI. Invest. Ophthalmol. Vis. Sci. 33:3500–3506 (1992).
36. N. Alikacem, T. Yoshizawa, K. D. Nelson, and C. A. Wilson. Quantitative MR imaging study of intravitreal sustained release of VEGF in rabbits. Invest. Ophthalmol. Vis. Sci. 41:1561–1569 (2000).
37. H. Kim, M. R. Robinson, M. J. Lizak, G. Tansey, R. J. Lutz, P. Yuan, N. S. Wang, and K. G. Csaky. Controlled drug release from an ocular implant: an evaluation using dynamic 3-dimensional magnetic resonance imaging. Invest. Ophthalmol. Vis. Sci. 45:2722–2731 (2004).
38. A. F. Holleman and E. Wiberg. Inorganic Chemistry, Academic Press, New York, 2001, Ch. 20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Molokhia, S. & Jeong, EK. Assessment of Subconjunctival Delivery with Model Ionic Permeants and Magnetic Resonance Imaging. Pharm Res 21, 2175–2184 (2004). https://doi.org/10.1007/s11095-004-7669-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-7669-3