An outbreak of a new coronavirus disease (COVID-19) in China in December 2019 became the epicenter for the spread of a global pandemic. The SARS-CoV-2 coronavirus causes a cascade of respiratory diseases similar to severe acute respiratory syndrome (SARS). Currently, there is no effective, specific, and safe treatment for COVID-19 to suppress the virus in the human body. The present study searched for pharmacological substances with antiviral activity for possible drug repositioning based on experimental and theoretical information in a series of publications on in vitro assays of agents against SARS-CoV-2. An analysis identified 46 well-known pharmaceutical substances that could be used for drug repositioning to create a therapy for COVID-19.
Similar content being viewed by others
The first cases of COVID-19 (COrona VIrus Disease 2019), which is caused by the coronavirus SARS-CoV-2, were recorded at the end of 2019 in Wuhan, People’s Republic of China. Subsequent spread of the infection over the whole world during several months led to the announcement by the WHO of a pandemic. The lethality from pathologies related to the infection varied from 2 to 6% despite all efforts including the use of known antiviral drugs to treat COVID-19 and associated complications [1].
The Ministry of Health of the RF developed temporary recommendations including a list of drugs that could be used to treat COVID-19 [2]. A list of three drugs, i.e., favipiravir, a flu virus RNA-dependent RNA-polymerase inhibitor; umifenovir, an antiviral agent; and hydroxychloroquine, an anti-malaria drug; is included in the current eighth version. It is noteworthy that the combination of the broad-spectrum antibiotic azithromycin and hydroxychloroquine enhanced the antiviral effect of the latter according to results from a single clinical trial. However, existing clinical data are contradictory. For example, use of hydroxychloroquine was demonstrated to be ineffective and unsafe for therapy of COVID-19 [3]. According to published data, a specific drug for etiotropic therapy of COVID-19 does not yet exist. Objective evidence for clinical use of the above drugs is lacking. Their mechanism of action on SARS-CoV-2 is not yet clear. Also, serious side effects occur and limit the use of the recommended medicines. For example, hydroxychloroquine exhibits cardiotoxicity. Long-term administration of it can lead to sudden cardiac death [4]. All this suggests that new specific drugs and additional molecular targets that could be used for COVID-19 therapy need to be discovered.
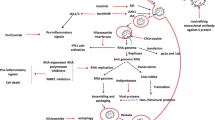
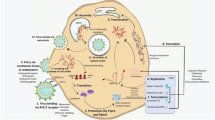
The molecular principles of the interaction of the virus with the human body have already been proposed and proteins that could be considered targets for antiviral therapy have been determined because of the rapid sequencing of the SARS-CoV-2 genome. The SARS-CoV-2 structural proteins that are most promising for drug development include the S-protein (Spike-protein), which is a part of the virus membrane shell and is responsible for its binding to the host cell through interaction with angiotensin-converting enzyme 2 (ACE2). S-protein must be activated and ACE2 must be proteolytically cleaved for fusion to occur. These processes are carried out in the human body by transmembrane protease serine TMPRSS2 [5], which itself is just as important a target for COVID-19 therapy.
The main protease Mpro (3CLpro) is necessary for release of various nonstructural viral proteins NSP 1 – 16 from polyproteins PP1A and PP1B and plays an important role in the life cycle of SARS-CoV-2. Mpro has no homologs among human enzymes so its inhibitors should not exhibit cytotoxic action on host cells, which is an important factor in choosing a potential target [6]. Another enzyme, papain-like protease (PLpro), is necessary to cleave the polyproteins. PLpro blocks production of proinflammatory cytokines such as IFN-β and chemokines CXCL10 and CCL5 via hydrolysis of ubiquitin and the protein interferon-stimulated gene 15 (ISG15), which are important elements of the inherent immune response to viral infection. This disrupts the regulation of signal cascades with subsequent death of surrounding uninfected cells. PLpro has several advantages as a target for antiviral therapy because its inhibitors can not only suppress virus replication but also reduce the death of surrounding uninfected cells while preserving the level of proinflammatory cytokines required to launch the inherent immune response [7]. RNA-dependent RNA-polymerase (RdRp) catalyzes viral RNA synthesis and is another functional protein that could be used as a therapeutic target to develop synthetic nucleotide drugs [8].
Because new drug development is a complicated, prolonged, and costly process, repurposing (repositioning) of drugs could accelerate the search for an antiviral therapy after outbreaks of new diseases such as COVID-19. Repositioning or repurposing of drugs implies a search for a new application that goes beyond the limits of the initial medical indications for an existing therapeutic drug. This procedure not only accelerates new drug development and reduces financial expenditures but also reduces the risk of an unfavorable result in various stages of preclinical and clinical trials. However, additional information that may include evidence of unexpected side effects, data on the interaction with the new target, etc. must be obtained to repurpose a therapeutic drug [9].
It is noteworthy that the first approaches to repurposing drugs for COVID-19 therapy using information about the in vitro activity were previously described for drugs aimed at the related coronaviruses SARS-CoV and MERS-CoV [10]. However, those results could not be directly used based on analogy. Additional experimental assessment of the activity of specific pharmaceutical substances directly against the SARS-CoV-2 virus were required for successful repurposing.
Currently, several dozen articles on in vitro testing of various drugs against SARS-CoV-2 have been published. However, different procedures and materials, including virus strains and cell cultures, were used in each study so that the obtained results were difficult to compare.
The goal of the present work was to analyze recent attempts to reposition drugs to seek an effective therapy for COVID-19 by analyzing the corresponding studies by various research groups to study the in vitro activities of existing drugs against SRAS-CoV-2.
Experimental Part
The studied drugs are used for therapy of various pathologies and are approved by the U. S. Food and Drug Administration (FDA), European Medicines Agency (EMA), Pharmaceuticals and Medical Devices Agency of Japan (PMDA), and regulatory bodies of other countries.
A total of 13 publications in which from 1 to >5,000 drugs were tested using various methods for assessing the antiviral activity of the compounds in cell cultures were analyzed by us.
An analysis of the publications showed that the studies were conducted using various strains of the SARS-CoV-2 virus, i.e., USA-WA1, which was isolated from the patient with the first recorded case of COVID-19 in the USA [11,12, – 13]; VIC01, which was obtained from the first recorded patient in Australia [14]; one of the first clinical isolates WIV04, which was isolated in 2019 in China [15]; KCDC03, which was found in a patient in South Korea [16]; BavPat1, which was obtained from a patient from Germany even before the epidemic spread to Europe [17]; HKU-001a, a clinical isolate from a patient from Hong Kong [18]; SZTH-003, which was isolated from a patient from China [19]; and IDF0372, which was obtained from the first patient in France who arrived from Wuhan [20].
The Vero cell line from kidney epithelial cells of an African green monkey [14, 16, 21] and its VeroE6 modification [12, 15, 17, 18, 20,21, – 22] were selected for testing in most studies. Human cell lines were used in three studies, i.e., Caco-2 (human colorectal adenocarcinoma cells) [19], Huh7 (hepatocellular carcinoma cells) [11], and HRCE (renal cortical epithelial cells) [13]. Also, BHK-21 baby hamster kidney fibroblasts expressing human ACE2 were also used in one of the works [21].
Six studies tested from 1 to 48 compounds. This allowed the activity of each compound to be analyzed in detail using one or several methods. The activities of the drugs were evaluated based on several experimental approaches, e.g., polymerase chain reaction with reverse transcription (RT-PCR) [11, 14, 15, 20, 22, 23], determination of antiviral activity by evaluating inhibition of a cytopathic effect caused by the virus [16], determination of expression of viral nucleocapsid protein (N-protein) using immunofluorescence analysis [15, 16, 20, 22], and counting the number of viable cells in culture by quantitative assay of the ATP concentration [12].
Atwo-stage screening system that allowed the number of analyzed compounds to be reduced in steps was used in studies where >100 compounds were evaluated in vitro. In one instance, the number of viable cells in the first stage was evaluated by analyzing their metabolic activity after infection and subsequent treatment with the tested compounds at a concentration of 10 μM. Umifenovir was used as the control compound to calculate the quantitative inhibition. The quantitative half-maximum effective concentration was measured using RT-PCR for those compounds that exhibited activity comparable to that of umifenovir at the given concentration [17]. In another instance, the activities of the compounds at a concentration of 10 μM were evaluated in the first stage using immunofluorescence analysis. Active compounds were evaluated for the ability to reduce the viral load using RT-PCR and determining the quantitative EC50 values [18]. In a third instance, all compounds at a concentration of 10 μM were evaluated in the first stage for the ability to inhibit a cytopathic effect caused by the virus. The quantitative half-maximum inhibitory concentrations for compounds identified as the most active that did not exhibit cytotoxicity were determined in the second stage [19].
A research group from China developed a method for assessing the antiviral activity of drugs by an immunofluorescence method using a pseudovirus consisting of vesicular stomatitis virus (VSV) bearing truncated S-protein of SARS-CoV-2 virus. This pseudovirus was shown to be capable of penetrating a cell analogously to the native coronavirus. According to the researchers, advantages of this system were its safety for personnel and the reduced time to identify the virus and the corresponding changes within the cell [24]. This approach allowed the discovery of 44 active compounds but only 13 compounds that were not cytotoxic against uninfected cell culture. The reduction of the cytopathic effects was evaluated further using a clinical isolate of SARS-CoV-2 [21].
Use of a modified cell painting method for evaluating morphological changes in cell cultures after infection of them by SARS-CoV-2 and subsequent treatment with the tested compounds was reported. Changes of cellular components and organelles were assessed using five different dyes. Morphological changes of the cells before and after treatment were subsequently compared using a patented algorithm for automatic processing of microscope images of cellular structures. Each of the tested compounds was assigned a quantitative estimate in the range from –1, signifying the lack of an antiviral effect, to 1, the ability to inhibit virus replication [13].
Table 1 summarizes the characteristics of the above methods used in the analyzed publications, the types of cell lines, the number of tested drug substances, and the corresponding references.
Research groups used three approaches to select drugs for in vitro testing. The first approach was based on already available data on the drug activity against related viruses, in particular, SARS-CoV and MERS-CoV. The activities of ribavirin, penciclovir, nitazoxanide, nafamostat, and chloroquine, which are approved by the FDA, and remdesivir and favipiravir, broad-spectrum antiviral agents, were evaluated. Activity against SARS and MERS viruses was previously demonstrated for ribavirin, nitazoxanide, chloroquine, nafamostat, and remdesivir; against Ebola virus, for favipiravir. Penciclovir is a nucleoside analog that is used to treat herpes infections [15]. Ivermectin is used to treat parasitic infections and was selected for the study based on data for its activity against various RNA-viruses [14]. Sixty-eight drugs that were already demonstrated in previous studies to be active against SARS-CoV and MERS-CoV were evaluated for antiviral activity against SARS-CoV-2 [12, 16]. A method for choosing tested drugs based on their inhibition of human coronavirus OC43 that causes seasonal URTI was reported. The virus strain selected for the first stage of the studies belongs to the same â-coronavirus family as SARS-CoV-2 and is the most common virus in the world. About 1700 therapeutic drugs approved by the FDA were tested. Of those, 26 were active against OC43 and were subsequently evaluated against one of the clinical strains of SARS-CoV-2 [22].
The second approach was based on existing data for the molecular mechanisms of interaction of coronaviruses with the host cell. Therefore, drugs that presumably could affect human proteins necessary to the SARS-CoV-2 life cycle were selected for the analysis. The activity of the anti-inflammatory agent auranofin, which is used to treat rheumatoid arthritis, was checked. Its proposed mechanism of action involves the inhibition of redox enzymes, induction of endoplasmic reticulum stress, and subsequent activation of the unfolded protein response (UPR), which leads to apoptosis of the cells. According to the researchers, the action of auranofin on redox enzymes could affect folding of SARS-CoV-2 viral proteins [11]. The drugs camostat and nafamostat were selected in an analogous manner. They are inhibitors of human serine proteases such as trypsin and complement component C1r. Presumably, these drugs also inhibit transmembrane protease serine TMPRSS2, which is a promising target for COVID-19 therapy [23]. A special analysis identified protein–protein interactions of SARS-CoV-2 and host cells and was used to select the tested drugs. The obtained results were used to determine human proteins that were drug targets and 76 chemical compounds interacting with them that could potentially inhibit virus replication [20].
The third approach for selecting drugs for the studies involved an attempt to include as many drugs as possible. According to the researchers, this allowed activity against SARS-CoV-2 to be found for compounds with various mechanisms of action and therapeutic indications. This could be useful for identifying new classes of drugs inhibiting coronavirus replication. However, the large number of tested drugs hindered a detailed evaluation of each of them. Therefore, the researchers first evaluated the cytotoxicity of each tested drug against the used cell lines to avoid false-positive results. This approach was used in five studies in which 1670, 1520, 1528, 5632, and 1403 compounds were tested against SARS-CoV-2 [13, 17,18,19,20]. Sets of compounds for testing included drugs approved by the FDA, EMA, PMDA, and other regulatory bodies in addition to drugs withdrawn from the market and compounds in various stages of clinical trials. The selected drugs included those exhibiting inhibitory active against other coronaviruses and those for which such activity was not previously reported.
It is noteworthy that four research groups [12, 16, 17, 19] published complete information about all studied compounds including inactive ones in the ChEMBL free-access database. Using these data, we counted 935 tested compounds that overlapped in these studies. However, none of these sets of compounds overlapped completely the others. Information on inactive compounds was unavailable for the other analyzed publications.
Thus, in vitro testing of the various drugs in the publications analyzed by us used various materials and methods.
Results and Discussion
Testing results obtained by various research groups were summarized using the sets of compounds from the above publications. Information about the initial therapeutic indications and mechanisms of action of 46 compounds for which inhibition of SARS-CoV-2 replication in vitro was demonstrated in two or more studies was analyzed. Table 2 lists the names and structural formulas of the drugs, the mechanisms of action, therapeutic indications, and activities against SARS-CoV-2 for these compounds. Quantitative values are shown in the table if they were given in the analyzed publications because calculated and measured activity data were not available for all identified compounds.
Table 2 divides the selected compounds into two groups depending on the type of virus used for the testing. The SARS-CoV-2 group includes test results for compounds that were shown to be active in two and more studies using the authentic coronavirus strain. Drugs for which the activity was demonstrated in studies using pseudovirus and was confirmed only in one publication using a clinical strain of SARS-CoV-2 are included in the Pseudovirus group.
Drugs selected for in vitro testing included four estrogen receptor modulators, i.e., tamoxifen, raloxifene, bazedoxifene, and toremifene; four dopamine receptor antagonists, i.e., asenapine, fluphenazine, thioridazine, and trifluoperazine; three Na+-K+-ATPase inhibitors, i.e., digitoxin, digoxin, and ouabain; two protein kinase inhibitors, i.e., sorafenib and regorafenib; and two proteinase serine inhibitors, i.e., camostat and nafamostat.
The most representative therapeutic groups were antitumor drugs (gemcitabine, raloxifene, tamoxifen, sorafenib, omacetaxine mepesuccinate, regorafenib, and toremifene); antiarrhythmics (digitoxin, digoxin, fendiline, ouabain, and dronedarone); antipsychotics (asenapine, thioridazine, trifluoperazine, and fluphenazine); antibacterials (hexachlorophene, salinomycin, cetylpyridinium, and Methylene Blue); and antimalarial agents (amodiaquine, chloroquine, hydroxychloroquine, and mefloquine), two of which (hydroxychloroquine and chloroquine) also possessed anti-inflammatory activity. It is worth mentioning that the selected compounds included three (remdesivir, tilorone, and umifenovir) with different mechanisms of action that are used to treat various viral diseases; three anthelmintics (oxyclozanide, niclosamide, and ivermectin); three immunosuppressants (cyclosporin, sirolimus, and mycophenolic acid); two drugs for pancreatitis therapy (nafamostat and camostat), two antiallergic and antiasthmatic agents (ciclesonide and clemastine); two drugs for postmenopausal disorder therapy (bazedoxifene and raloxifene); two antiprotozoal agents (nitazoxanide and monensin); and one compound each from the antihypertensive (candesartan), antidepressant (vortioxetine), antidiarrheal (loperamide), respiratory stimulator (almitrine), and antileprotic classes (clofazimine).
Two antimalarial drugs, chloroquine and mefloquine, were used for therapy of COVID-19 [25] and were active in vitro in six and four studies, respectively. It is worth noting than the use of these drugs for COVID-19 therapy was allowed in an earlier version of Recommendations of the RF Ministry of Health although their use was stopped because of results from clinical trials in Russia. Several proposed mechanisms of action of these drugs against SARS-CoV-2 were suggested. One of them was prevention of virus endocytosis in the host cell [26]. Remdesivir was reported to be an active antiviral drug in four publications and was also included in the list of recommended drugs for COVID-19 therapy in the USA [27]. Remdesivir is an antiviral drug of the synthetic nucleoside derivative class. Its active metabolite, remdesivir triphosphate, presumably inhibits viral RNA-dependent RNA-polymerase [28]. Nafamostat, which is approved in Japan for treating pancreatitis and disseminated intravascular blood clotting syndrome, was confirmed to be active against SARS-CoV-2 in three studies. A possible mechanism of action of this drug was related to inhibition of transmembrane protease serine TMPRSS2, which is involved in penetration of coronavirus into the host cell [24]. Studies in vitro demonstrated that nafamostat and camostat inhibited TMPRSS2 at nanomolar concentrations [29]. Tilorone, an interferon inductor that is used in Russia and the CIS countries to treat and prevent a broad spectrum of viral infections, was found to be active against SARS-CoV-2 [30]. Clinical trials of this drug against COVID-19 are currently under way in Ukraine [31].
Sixteen of the 46 compounds given in Table 2 are in clinical trials aimed at COVID-19 therapy. They include almitrine, camostat, chloroquine, ciclesonide, hydroxychloroquine, cyclosporin, mefloquine, remdesivir, nafamostat, nitazoxanide, tamoxifen, tilorone, sirolimus, ivermectin, umifenovir, and niclosamide [31, 32]. It is noteworthy that clinical trials for treating COVID-19 have already been conducted for the antimalarial drug hydroxychloroquine. Hydroxychloroquine was initially considered a promising drug against coronavirus infection but did not demonstrate efficacy in clinical trials in comparison with standard symptomatic therapy. Therefore, these trials were halted in many countries [3]. Almitrine was studied in patients with COVID-19 as a respiratory stimulant to reduce hypoxemia with acute respiratory distress syndrome. Most patients receiving almitrine required additional therapy. An antiviral effect of this drug in the human body at the used therapeutic concentration was not observed despite a reduction of hypoxemia and demonstrated in vitro activity against SARS-CoV-2 [33]. The glucocorticosteroid ciclesonide, which is used to treat asthma and allergic rhinitis, was tested in other studies to reduce hypoxemia. The dynamics of the change of clinical indicators resulting from the therapy gave a positive evaluation for the use of this drug [34]. Clinical trial data for the other 30 drugs given in Table 2 were not found by us.
Conclusion
Drugs that were confirmed in several independent studies to inhibit in vitro replication of various strains of SARS-CoV-2 and are currently being studied for repositioning were examined by us. Both separate drugs exhibiting activity against coronavirus at micromolar concentrations, e.g., clofazimine and salinomycin, and therapeutic drug classes, several of which possessed antiviral activity, e.g., estrogen receptor modulators and cardiac glycosides inhibiting Na+-K+-ATPase, were found among the identified compounds. Pharmacological compounds tested in publications analyzed by us were promising candidates for treating the new coronavirus infection because their pharmacokinetic profiles and side effects were previously studied. These data could significantly reduce the risk for patients and the finances and time expended to create specific therapy against the SARS-CoV-2 virus. Clinical trials for COVID-19 therapy were conducted or are being conducted for 16 of the examined compounds. However, clinical data for most of the studied drugs were not found by us.
Further preclinical and clinical trials of tested drugs and those that exhibited antiviral activity in vitro are needed to determine their associated targets and specific pharmacotherapeutic effects with respect to the human body during COVID-19 therapy.
References
WHO Coronavirus Disease (COVID-19) Situation Report 209; https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200816-covid-19-sitrep-209.pdf?Sfvrsn=5dde1ca2_2.
RF Ministry of Health Recommendations, ver. 8; https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/051/777/original/030902020_COVID-19_v8.pdf.
WHO Review: The cardiotoxicity of antimalarials; https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicityreport-session2.pdf.
Y. F. Tu, C. S. Chien, A. A. Yarmishyn, et al., Int. J. Mol. Sci., 21(7), 2657 (2020).
L. Zhang, D. Lin, X. Sun, et al., Science, 368(6489), 409 – 412 (2020).
Y. M. Baez-Santos, S. E. St John, and A. D. Mesecar, Antiviral Res., 115, 21 – 38 (2015).
Y. Gao, L. Yan, Y. Huang, et al., Science, 368(6492), 779 – 782 (2020).
V. Poroikov and D. Druzhilovskiy, in: In Silico Drug Design: Repurposing Techniques and Methodologies, K. Roy (ed.), Academic Press, New York (2019), pp. 3 – 17.
J. M. Ulm and S. F. Nelson, Transboundary Emerging Dis., (2020); doi: https://doi.org/10.1111/tbed.13710.
H. A. Rothan, S. Stone, J. Natekar, et al., Virology, 547, 7 – 11 (2020).
S. Weston, C. Coleman, R. Haupt, et al., J. Virol., 94(21), e01218 – 20 (2020); doi: https://doi.org/10.1128/JVI.01218-20.
K. Heiser, P. F. McLean, C. T. Davis, et al., bioRxiv [Preprint] (2020); https://doi.org/10.1101/2020.04.21.054387.
L. Caly, J. D. Druce, M. G. Catton, et al., Antiviral Res., 178, 104787 (2020).
M. Wang, R. Cao, L. Zhang, et al., Cell Res., 30(3), 269 – 271 (2020).
S. Jeon, M. Ko, J. Lee, et al., Antimicrob. Agents Chemother., (2020); doi: https://doi.org/10.1128/AAC.00819-20.
F. Touret, M. Gilles, K. Barral, et al., Sci. Rep., 10(1), 13093 (2020).
S. Yuan, J. F. W. Chan, K. K. H. Chik, et al., Pharmacol. Res., 159, 104960 (2020).
B. Ellinger, D. Bojkova, A. Zaliani, et al., Research Square [Preprint] (2020); doi: https://doi.org/10.21203/rs.3.rs-23951/v1.
D. E. Gordon, G. M. Jang, M. Bouhaddou, et al., Nature, 583, 459 – 468 (2020).
H.-L. Xiong, J.-L. Cao, C.-G. Shen, et al., bioRxiv [Preprint] (2020); https://doi.org/10.1101/2020.06.05.135996.
X. Xiao, C. Wang, D. Chang, et al., bioRxiv [Preprint] (2020); https://doi.org/10.1101/2020.07.06.188953.
M. Hoffmann, S. Schroeder, H. Kleine-Weber, et al., Antimicrob. Agents Chemother., 64(6), e00754 – 20 (2020).
H.-L. Xiong, Y.-T. Wu, J.-L. Cao, et al., bioRxiv [Preprint] (2020); https://doi.org/10.1101/2020.04.08.026948.
Recommendations of RF Ministry of Health, ver. 7; https://static-0.rosminzdrav.ru/system/attachments/attaches/000/050/584/original/03062020%D0%9CR_COVID-19_v7.pdf.
S. Tripathy, B. Dassarma, S. Roy, et al., Int. J. Antimicrob. Agents, 106028 (2020).
FDA Remdesivir; https://www.fda.gov/media/137566/download.
R. T. Eastman, J. S. Roth, K. R. Brimacombe, et al., ACS Cent. Sci., 6(5), 672 – 683 (2020).
J. H. Shrimp, S. C. Kales, P. E. Sanderson, et al., bioRxiv [Preprint] (2020); https://doi.org/10.1101/2020.06.23.167544.
R. Barthelemy, P.-L. Blot, A. Tiepolo, et al., Chest, (2020); doi: https://doi.org/10.1016/j.chest.2020.05.573.
K. Nakajima, F. Ogawa, K. Sakai, et al., Mayo. Clin. Proc., 95(6), 1296 – 1297 (2020).
Acknowledgments
The work was financially supported by RFBR Project No. 20-04-60285.
Author information
Authors and Affiliations
Corresponding author
Additional information
Notes:
IC50 is the half-maximum inhibitory concentration, μM;
EC50 is the half-maximum effective concentration, μM;
Inhibition is the value in percent of virus replication inhibition based on cell viability assay;
Inh. Index (inhibition index) is a quantitative parameter of virus replication inhibition based on a cell viability assay as compared to umifenovir preparation [17];
Score is a quantitative estimate of virus replication inhibition ranging from –1 to +1 calculated using a patented algorithm [13].
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 10, pp. 7 – 14, October, 2020.
Rights and permissions
About this article
Cite this article
Savosina, P.I., Druzhilovskii, D.S. & Poroikov, V.V. COVID-19: Analysis of Drug Repositioning Practice. Pharm Chem J 54, 989–996 (2021). https://doi.org/10.1007/s11094-021-02308-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02308-0