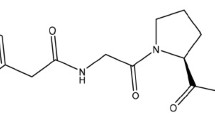
A series of C-substituted N-phenylacetylglycylprolines were synthesized. Their structure-activity (antihypoxic) relationship was studied in normobaric hypoxia tests at i.p. doses of 0.1 – 1 mg/kg as a function of the C-substitution. The amide and ethyl ester of N-phenylacetylglycyl-L-proline exhibited antihypoxic activity and could cyclize in biological fluids to form cycloprolylglycine that was identical to the endogenous peptide with antihypoxic, neuroprotective, and other types of neuropsychotropic activity. The antihypoxic effect was stereospecific because the D-enantiomers were inactive. These compounds at concentrations of 10–7 – 10–5 M also exhibited neuroprotective activity for SH-SY5Y human neuroblastoma cell culture with 6-hydroxydopamine neurotoxicity. The methylamide and analogous free acid of N-phenylacetylglycylproline could not be cyclized into cycloprolylglycine and were inactive. It was concluded that the activity of the substituted glyprolines was associated with their conversion into cycloprolylglycine.


Similar content being viewed by others
References
T. A. Gudasheva, S. S. Boyko, V. Kh. Akparov, et al., FEBS Lett., 391, 149 – 152 (1996).
T. A. Gudasheva, R. U. Ostrovskaya, S. S. Trofimov, et al., Byull. Eksp. Biol. Med., 116(10), 411 – 413 (1999).
T. A. Gudasheva, M. A. Konstantinopol’skii, R. U. Ostrovskaya, and S. B. Seredenin, Byull. Eksp. Biol. Med., 131(5), 547 – 550 (2001).
S. B. Seredenin, T. A. Gudasheva, S. S. Boiko, et al., Byull. Eksp. Biol. Med., 133(4), 417 – 419 (2002).
K. N. Kolyasnikova, T. A. Gudasheva, G. A. Nazarova, et al., Eksp. Klin. Farmakol., 75(9), 3 – 6 (2012).
P. Yu. Povarnina, K. N. Kolyasnikova, S. V. Nikolaev, et al., Byull. Eksp. Biol. Med., 160(11), 600 – 603 (2015).
T. A. Gudasheva, N. I. Vasilevich, N. N. Zolotov, et al., Khim.-farm. Zh., 25(6), 12 – 16 (1991); Pharm. Chem. J., 25(6), 363 – 367 (1991).
T. A. Gudasheva, V. V. Grigor’ev, K. N. Kolyasnikova, et al., Dokl. Akad. Nauk, 471(1), 106 – 108 (2016).
T. A. Gudasheva, K. N. Kolyasnikova, T. A. Antipova, et al., Dokl. Akad. Nauk, 469(4), 492 – 495 (2016).
T. A. Gudasheva, K. N. Kolyasnikova, E. A. Kuznetsova, et al., Khim.-farm. Zh., 50(11), 3 – 8 (2016); Pharm. Chem. J., 50(11), 705 – 710 (2011).
G. W. Anderson, J. E. Zimmerman, and F. M. Callahan, J. Am. Chem. Soc., 89, 5012 – 5017 (1967).
E. Baumann, Ber. Dtsch. Chem. Ges., 19, 3218 – 3222 (1886).
M. Brenner and W. Huber, Helv. Chim. Acta, 36, 1109 – 1115 (1959).
T. A. Voronina, R. U. Ostrovskaya, and T. L. Garibova, “Methodical recommendations for preclinical trials of drugs with nootropic activity,” in: Handbook for Preclinical Drug Trials [in Russian], A. N. Mironov (ed.), Izd. FGBU NTsEMSP, Ministry of Health and Social Development of Russia, Moscow (2012), Vol. 1, pp. 276 – 296.
T. A. Gudasheva, T. A. Voronina, R. U. Ostrovskaya, et al., Eur. J. Med. Chem., 31, 151 – 157 (1996).
K. Riveles, L. Z. Huang, and M. Quik, Neurotoxicology, 29(3), 421 – 427 (2008).
G. R. Jackson, K. Werrbach-Perez, E. L. Ezell, et al., Brain Res., 592(1–2), 239 – 248 (1992).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 6, pp. 13 – 17, June, 2018.
Rights and permissions
About this article
Cite this article
Kolyasnikova, K.N., Kuznetsova, E.A., Nikolaev, S.V. et al. Structure–Activity (Antihypoxic) Relationship in a Series of Substituted Glyprolines. Pharm Chem J 52, 501–505 (2018). https://doi.org/10.1007/s11094-018-1848-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1848-8