Abstract
Hollow particles have attracted considerable attention owing to their unique properties. In this study, hollow monoclinic zirconia particles were directly synthesized from inkjet droplets of a zirconyl hydroxychloride aqueous solution via atmospheric-pressure plasma processing. Hollow structures with craggy surfaces were obtained in the plasma at gas temperatures above 1000 K. The steep solvent evaporation rate induced by the localized high-energy reaction field of the atmospheric-pressure plasma may have induced solute condensation near the droplet surface and contributed to the formation of hollow particles. The average diameter of the synthesized particles was ~ 3 μm, while their size distribution was narrow (coefficient of variation: 0.06–0.10). The high reproducibility of the synthesized particles was attributed to the small variations in inkjet droplet size. The proposed method enables the rapid synthesis of hollow particles of various inorganic materials, while controlling their number and composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hollow particles have attracted considerable attention for applications in catalysts, batteries, drug delivery systems, bioencapsulation, and gas storage owing to their high volume-to-surface ratio, low density, thermal insulation, and ability to enclose other substances inside particles [1,2,3,4]. Various methods have been developed to synthesize hollow particles, such as colloidal template methods [5], emulsion polymerization [6], and spray pyrolysis [7]. Particle synthesis processes that start from liquid droplets (e.g., spray pyrolysis) are promising methods to synthesize hollow particles because of their ability to synthesize various inorganic materials with low impurities in the synthesized particles, low operating costs, and large yields [8]. In conventional spray pyrolysis methods, the raw material solution is atomized by an ultrasonic atomizer and introduced into the high-temperature region by a carrier gas to directly produce the desired particles by reacting with the gas or pyrolysis. Hollow particles are synthesized owing to the local supersaturation of the solute near the droplet surface due to the evaporation of the solvent, and the precipitation of the solute as a solid phase (solid shell) [8,9,10]. The solid shell formation near the droplet surface reduced the heat loss due to evaporation, resulting in an increase in the droplet temperature. When the internal particle temperature reaches the boiling point, a bubble forms in the droplet, which may also contribute to the formation of hollow particles. Experiments have revealed that the steeper the evaporation rate that increases the solute concentration gradient within the droplet, the more likely that hollow particles are synthesized [8, 9]. Although hollow particles can be synthesized by spray pyrolysis, the particles produced exhibit poor monodispersity because of the wide variations in the droplet size generated by ultrasonic atomizers. The coefficient of variation (CV), the ratio of the standard deviation (σ) to the mean (x̄), of the particles synthesized by the spray pyrolysis method was found to exceed 0.2 owing to the variations in mist droplet size [11, 12].
In this study, hollow particles of zirconium dioxide (i.e., zirconia) with diameters of several micrometers were synthesized via an atmospheric-pressure nonequilibrium plasma system with inkjet droplets. Zirconia is a well-studied functional material because of its physical and chemical properties. Its applications include coatings [13, 14], ionic conductors [15], and oxygen sensors [16]. Although zirconia particles, including hollows, have been synthesized by the spray pyrolysis method [8, 9, 17,18,19,20], the proposed system is expected to have advantages owing to (i) the narrow size distribution of the initial inkjet droplets and (ii) the localized high-energy reaction fields provided by the atmospheric-pressure nonequilibrium plasma. The CV of the droplet diameters generated by an inkjet device (< 0.006 [21]) is considerably smaller compared with the droplets generated by an atomizer (> 0.2 [22, 23]), resulting in the excellent reproducibility of the synthesized particles [24, 25]. In this study, an atmospheric-pressure nonequilibrium plasma system generated by a 450 MHz ultra-high-frequency (UHF) power supply was utilized. This system can instantly inject a droplet into an environment with a gas temperature above 1000 K. Furthermore, the atmospheric-pressure plasma at high frequency is nearly temporary stable and has a high time-averaged plasma density (> 1019 m− 3) [26, 27]. Such a local high-temperature environment, coupled with energy deposition by high-energy electrons (> 1 eV), ions accelerated by a negative floating potential around the droplet, and recombination reactions between electrons and ions, can rapidly evaporate the solvent from the droplet surface [26, 28], resulting in local solute condensation near the droplet surface. The direct solid-phase formation reaction induced by the radicals supplied by the plasma could further promote solid-phase generation near the droplet surface, which would be ideal for the rapid and easy synthesis of hollow particles.
Experimental
Materials
Zirconyl hydroxychloride (ZrO(OH)Cl: ZHC) aqueous solution (Daiichi Kigenso Kagaku Kogyo Co., Ltd., ZC-2) and deionized water (Fujifilm Wako Pure Chemical Corp.) were mixed to prepare 10 mM solutions. Ar gas (> 99.9995%) was purchased from TAIYO NIPPON SANSO Corp.
Preparation Method
Figure 1(a) shows a schematic of the experimental setup, which is similar to that reported in our previous study [24]. An inkjet head and a pair of copper electrodes were placed in a polytetrafluoroethylene (PTFE) casing, with quartz windows on the front and back surfaces. The copper electrodes formed a parallel-plate discharge gap with an interelectrode spacing of 0.5 mm, and vertical and horizontal lengths of 10 and 1 mm, respectively. Cooling water at 5 °C was allowed to flow inside each electrode to stabilize the discharge during experiment. Droplets of the ZHC solution were ejected from the inkjet head (Micro jet, IJHD-10) at a frequency of 50 Hz. The diameter of each droplet was measured as approximately 30 μm by a charge-coupled device (CCD) camera (Watec, WAT-902 H ULTIMATE), as shown in Fig. 1(b). Plasma was generated by applying an ultra-high-frequency wave of 450 MHz from a power supply (Tokyo Hi-power, RF50-450-P) to one of the copper electrodes. The output power was adjusted in the range of 35–50 W. The average gas temperature, estimated by fitting the OH emission spectrum to the theoretical spectrum (details are shown in the following section), was 800–1500 K. The argon gas flow rate was set as approximately 18 sccm. As observed in our previous study conducted using a similar system [26], the droplet velocity may be approximately equal to the gas velocity. Therefore, the residence time of the droplets or synthetic particles in the plasma was estimated to be in the range of 3–6 ms assuming ideal gas conditions. The synthesized particles were deposited on a carbon tape with a width and thickness of 10 mm and approximately 500 μm, respectively.
Characterization
The particles on the carbon tape were observed by a scanning electron microscope (SEM) (JEOL, JSM-IT500). The cross-sectional images of the particles observed by SEM included a focused ion beam (FIB) (JEOL, JIB-4700 F, and Oxford, EDS·EBSD) after FIB etching. The SEM images were analyzed using MATLAB (Mathworks, Inc., Natick, MA, USA) to determine the particle size distribution. The atomic composition of the particles was determined by energy-dispersive X-ray spectroscopy (EDS) (JEOL, JSM-IT500). The chemical structures of the samples were analyzed by Raman spectroscopy (JASCO, NRS-3100). Optical emission spectroscopy (OES) of the plasma was conducted using a spectrometer (Ocean Optics, HR4000) and an intensified CCD camera (Hamamatsu Photonics, C8484-05G01 and C7164-03) equipped with a spectrometer (HORIBA Scientific, iHR320).
Results and Discussion
OES Measurement
OES was used as a diagnostic tool for processing. Figure 2(a) shows the typical OES spectra of the plasma. Most of the emission lines identified in this study are listed in Table 1. The emission lines of the carrier gas Ar, atmospheric N2, and OH, H, and O originating mainly from the liquid droplets were clearly observable, whereas the emission lines attributed to Zr or Cl were hardly detectable. The bands around 618, 674, and 715 nm shown in Fig. 2(a) are the 2nd order diffraction signals of OH and N2 emissions. To evaluate the processing temperature, the OH rotational temperature (Tr) was estimated using a spectral fitting technique [29, 30]. Under high vapor concentration conditions, owing to the highly rotationally excited OH radicals produced by the direct dissociation of water molecules, fitting with a two-temperature model is commonly used, where low-temperature components are reported to be in equilibrium with the gas temperature [29,30,31]. In the present case, however, the analysis using the two-temperature model predicted that the best-match spectrum was almost a one-temperature spectrum with extremely few high-temperature components. Therefore, the one-temperature model is sufficient to estimate the rotational temperature. Figure 2(b) shows the fitting result using the one-temperature model at 50 W. The theoretical spectrum at Tr = 1460 K is in good agreement with the experimental spectrum. The same procedure was applied under the other experimental conditions. The environmental gas temperatures at output powers of 35, 45, and 50 W were 820, 1090, and 1460 K, respectively.
Time- and space-averaged optical emission spectra by applying a power of 50 W at the wavelengths (a) 350–800 nm and (b) 306–310 nm. In (b), the vertical axis representing the emission intensity is normalized by the peak at 390.1 nm; a theoretical spectrum of Tr = 1460 K is also indicated. The integration time was 50 ms in each figure
SEM Measurement
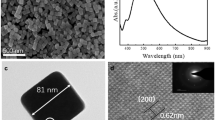
Figure 3 shows the SEM images of the particles synthesized from a 10 mM solution at output powers of 35, 45, and 50 W. The particles synthesized at 35 W were spherical in shape, whereas those synthesized at an output power above 45 W exhibited a craggy shape. Because of the craggy shape of the particles synthesized at 45 and 50 W, their equivalent projected area diameters (i.e., Heywood diameters; an index of the particle size) were analyzed from the projected area of the particle regions in each SEM image [36]. Figure 4 shows histograms of the equivalent projected area diameter distribution of the particles synthesized at each output power. The average diameters of the dominant particles synthesized at 35, 45, and 50 W were 2.70 ± 0.21, 2.88 ± 0.28, and 2.78 ± 0.18 μm, respectively. The particle size distributions were approximated using a normal distribution. The CV ranged from 0.06 to 0.10. Although the shapes of the particles synthesized at 45 and 50 W were not perfectly spherical, the substantial particle sizes were highly reproducible. The circularity (perimeter ratio) of the particles were obtained from the particle area projections depicted in the SEM images using the formula proposed by Cox (\(C=4\pi A/{P}^{2}\)) [37, 38], where \(C\), \(A\), and \(P\) are the circularity, projected area, and perimeter length of projected area, respectively. The average circularity of the particles synthesized at 35, 45, and 50 W were 0.94 ± 0.02, 0.93 ± 0.14, and 0.82 ± 0.13, respectively.
EDS Measurement
Figure 5(a)–(c) show the typical EDS spectra of the particles synthesized from the 10 mM solution at output powers of 35, 45, and 50 W. Carbon derived from the carbon tape of the substrate was detected in each sample. In the EDS spectra of the particles synthesized at 35 W, Cl atoms were detected in the raw material along with Zr and O atoms. Figure 5(d) shows the measured atomic ratio of Cl to Zr in the particles as a function of the output power. As the output power increased, the proportion of Cl atoms decreased. Cl atoms were not observed in the particles synthesized at 50 W, suggesting the sufficient reaction of the raw materials to synthesize ZrO2 at this output power.
Raman Spectroscopy
The chemical structures of the particles were evaluated by Raman spectroscopy. Figure 6 shows the Raman spectra of the particles synthesized at each output power with ZHC as the raw material. The ZHC exhibited a relatively broad spectrum with gentle peaks near 130 and 560 cm− 1. The particles synthesized at 35 W exhibited a broad spectrum; as the output power increased, several peaks became noticeable. These peaks were derived from the Raman-active phonon modes of Ag (181, 376 476, 557, and 635 cm− 1) and Bg (224 and 335 cm− 1), and clearly indicate the formation of crystalline monoclinic ZrO2, which is the stable crystalline phase at room temperature [39].
Cross-Sectional Observation of FIB-Etched Particles
To evaluate the shape of the synthetic particles, particularly whether these were hollow, the cross-sections of the particles etched by FIB were observed by SEM. Figure 7 shows the SEM images of the particles synthesized at output powers of 35, 45, and 50 W before and after FIB etching. Particles synthesized at 35 W with insufficient precursor decomposition were nearly spherical and exhibited a structure without cavities, as shown in Fig. 7(a). In contrast, the particles synthesized at 45 W exhibited a craggy surface and hollow interior (upper part of Fig. 7(b)), whereas a few were crushed and flattened (lower part of Fig. 7(b)). The particles synthesized at 50 W also exhibited a craggy surface, and almost all were hollow. For the particles synthesized at 50 W, where the zirconia synthesis reaction was sufficiently advanced, the shell thickness was approximately 200–500 nm. Assuming that the mass dissolved in one droplet is stored in one synthesized particle to form a perfectly spherical hollow particle, the relationships between the synthesized particle size, initial droplet size, and solution concentration can be expressed as follows:
where, \({r}_{\text{D}}\), \({C}_{0}\), \(M\), \(\rho\), \({r}_{\text{S}}\), and \({r}_{\text{H}}\) represent the initial droplet radius, initial solution concentration, molar mass of zirconia (123.218 g/mol), density of monoclinic zirconia (5.78 g/cm3), outer shell radius, and inner hollow radius of the synthesized particle, respectively. Taking the Heywood diameter presented in Fig. 4 as the outer shell diameter, the shell thickness (\({r}_{\text{S}}-{r}_{\text{H}}\)) was estimated approximately as 130 nm using the abovementioned equation. This is thinner than the thickness observed in Fig. 7(c); therefore, the outer shell of the particle synthesized at 50 W could attain a fine porosity. When the zirconia precursor precipitated as a solid phase and decomposed to form zirconia particles, a porous layer might form around the unreacted solid core as the reaction progressed [40].
Discussion on Hollow Particles Formation
The mechanism by which nonporous or hollow particles are synthesized from liquid droplets was discussed based on the concentration distribution of the solute inside the droplet [8,9,10]. First, the solute was uniformly distributed within the droplet immediately after it was dispensed. Upon the initiation of evaporation from the droplet surface, a concentration gradient occurred. The magnitude of the solute concentration gradient was dependent on the relative evaporation and diffusion rates of both the solvent to the surface and the solute to the droplet center. At slower evaporation rates, the solute concentration inside the droplet uniformly increased up to nearly the saturation concentration, while the solid phase was more likely to uniformly precipitate in the droplet. In contrast, if the evaporation of the solvent is rapid relative to the diffusion of the solute inside the droplet, then the concentration gradient inside the droplet increased and supersaturation could easily occur near the droplet surface. If the concentration at the center of the droplet is below the equilibrium saturation concentration (ES) when the outer edge of the droplet reaches the critical supersaturation concentration (CSS), then the solid phase is considered to precipitate only in the area near the droplet surface where the concentration exceeds the ES [8,9,10]. Notably, the final particle shape is not necessarily linked to the shape of the solid phase when it is first precipitated, as it may be affected by the difference in density between the precursor and final product. Another possible cause of hollow particle formation is bubble formation inside the droplet [10, 17]. When the solid phase precipitated only near the surface of the droplet, thereby forming a solid shell, it hindered solvent diffusion and decreased the evaporation rate, thus increasing the droplet temperature. When there is no solid shell in the droplet, the droplet temperature usually does not exceed the boiling point because the heat loss due to evaporation is balanced by the heat influx from the surroundings [26]. The existence of the solid shell also prevented solvent evaporation, causing a rapid increase in the liquid core temperature. If the liquid core temperature becomes higher than the boiling point of the solvent, then bubbles can be generated inside. In particular, in the case of a larger initial droplet size, bubble formation is more likely to occur because of the thicker shell that prevents solvent evaporation [10]. In either of the above hollow particle synthesis mechanisms, solid shell formation near the droplet surface is necessary. This is consistent with the experimental results showing that hollow particles were obtained at a higher power with gas temperatures above 1000 K.
At 45 W, both hollow and wrinkled flat particles were observed. The wrinkled flat shape could be attributed to the collapse of the originally hollow particles because the vapor trapped inside them was cooled by the outside air after passing through the plasma, thereby reducing the pressure inside the particles. As suggested by the EDS and Raman spectra, the particles synthesized at 45 W did not react sufficiently to synthesize hard crystalline zirconia from the raw material, and some particles were unable to maintain their hollow structures. The Heywood diameters estimated from the particle area projections depicted in the SEM images are likely to increase for flattened particles. Thus, the coefficient of variation regarding the Heywood diameters of the particles was maximized at 45 W. For the particles synthesized at 50 W, the synthesis and sintering of zirconia proceeded sufficiently to form a firm shell structure, essentially maintaining a hollow structure. In this case, inwardly curled holes were observed in numerous particles. These can be considered as holes created by ambient air entering from the outside when the internal pressure decreased after passing through the plasma.
The cause of the craggy surface structure of the particles synthesized at 45 and 50 W remains unknown. Until a solid shell is formed, the droplet is considered as spherical owing to the surface tension of water. Therefore, such a craggy surface structure should have occurred after the formation of the solid phase (solid shell). Previous research has speculated that during the drying of wet particles with a solid shell, numerous identical linear cylindrical holes were unevenly distributed in the solid shell, which allowed vapor to escape from the internal liquid core [41]. Notably, the rates of drying shrinkage, raw material decomposition, and sintering between these holes and other areas could vary, which may have led to the formation of particles with an irregular surface structure. Additionally, highly oxidizing species, such as O2−, H2O2, and OH−, are generated in the liquid phase in contact with the plasma. Such oxidizing species can act as oxygen sources for the direct synthesis of oxides, for example, zirconia [42]. In contrast to precipitation due to supersaturation of the solute concentration, heterogeneous nucleation on the droplet surface due to reactive species may contribute to the formation of irregularly shaped particles. In the literature, zirconia particles synthesized from ZHC by spray pyrolysis are predominantly spherical with smooth surfaces [8, 9, 17]. However, studies on the synthesis of particles with a rugged surface structure that remains hollow, as in the present case, are limited. We also synthesized particles from similar inkjet droplets using an electric furnace but could not obtain particles with a craggy surface morphology, even at temperatures above 1000 K (details are reported in the supplementary information). In our system adopting atmospheric-pressure plasma, the extremely steep progress of reactions such as evaporation, decomposition, and sintering, and possibly plasma-derived radical reactions, may contribute to these craggy shapes. Nevertheless, the mechanism remains to be elucidated. In the rapid gold nanoparticle synthesis process conducted with a similar plasma-droplet system, Maguire et al. estimated the equivalent energy dose rate transmitted to each droplet in the plasma as approximately 10− 2 kGy s− 1 (J kg− 1 s− 1) from the plasma-droplet volume ratio [43]. In our case, by assuming that the 50 W output power is entirely consumed in the plasma region (namely, upper limit estimation), the power received by each droplet is estimated as 10− 8–10− 5 W depending on the droplet diameter. This is equivalent to an energy dose rate of 101–102 kGy s− 1, which is three to four orders of magnitude larger than that estimated by Maguire et al. Our system constructed with UHF plasma, which can transmit large amounts of energy into each droplet, may enable the synthesis of micrometre-sized ceramic zirconia particles over a short plasma residence time of a few milliseconds.
Conclusion
Hollow zirconium dioxide particles were synthesized from a zirconyl hydroxychloride solution. Such hollow structures were obtained in the plasma with a higher power, such as gas temperatures above 1000 K. Solute condensation near the droplet surface owing to rapid solvent evaporation was expected to contribute to the formation of hollow particles. Although the hollow particles exhibited a craggy surface structure, the distribution of their Heywood diameters was narrow (CV: 0.06–0.10), indicating the high reproducibility of the particles synthesized via this method.
The proposed method using inkjet droplets enabled the synthesis of monodisperse particles of various inorganic materials with controlled sizes, shapes, and compositions. The inkjet device strictly controlled the droplet ejection by inputting an electric signal and could be easily scaled up using multiple nozzles. In the future, this process will enable high-throughput particle synthesis processes.
References
Wang X, Feng J, Bai Y et al (2016) Synthesis, Properties, and applications of Hollow Micro-/Nanostructures. Chem Rev 116:10983–11060. https://doi.org/10.1021/acs.chemrev.5b00731
Xia Y, Na X, Wu J, Ma G (2019) The Horizon of the Emulsion Particulate Strategy: Engineering Hollow particles for Biomedical Applications. Adv Mater 31:1801159. https://doi.org/10.1002/adma.201801159
Yasun E, Gandhi S, Choudhury S et al (2020) Hollow micro and nanostructures for therapeutic and imaging applications. J Drug Deliv Sci Technol 60:102094. https://doi.org/10.1016/j.jddst.2020.102094
Wichaita W, Polpanich D, Tangboriboonrat P (2019) Review on synthesis of Colloidal Hollow Particles and their applications. Ind Eng Chem Res 58:20880–20901. https://doi.org/10.1021/acs.iecr.9b02330
Caruso F, Caruso RA, Möhwald H (1998) Nanoengineering of Inorganic and Hybrid Hollow spheres by Colloidal Templating. Science 282:1111–1114. https://doi.org/10.1126/science.282.5391.1111
Noguchi S, Sato K, Yamamoto K, Kadokawa J (2019) Preparation of composite and hollow particles from self-assembled chitin nanofibers by Pickering emulsion polymerization. Int J Biol Macromol 126:187–192. https://doi.org/10.1016/j.ijbiomac.2018.12.209
Aghaali MH, Firoozi S (2019) Synthesis of nanostructured fcc/hcp hollow ni particles by ultrasonic spray pyrolysis and its dry reforming catalytic properties. Powder Technol 356:119–128. https://doi.org/10.1016/j.powtec.2019.08.023
Lenggoro IW, Hata T, Iskandar F et al (2000) An experimental and modeling investigation of particle production by spray pyrolysis using a laminar flow aerosol reactor. J Mater Res 15:733–743. https://doi.org/10.1557/JMR.2000.0106
Widiyastuti W, Wang W-N, Lenggoro IW et al (2007) Simulation and experimental study of spray pyrolysis of polydispersed droplets. J Mater Res 22:1888–1898. https://doi.org/10.1557/jmr.2007.0235
Jayanthi GV, Zhang SC, Messing GL (1993) Modeling of solid particle formation during solution Aerosol Thermolysis: the Evaporation Stage. Aerosol Sci Technol 19:478–490. https://doi.org/10.1080/02786829308959653
Tsumaki M, Shimizu Y, Ito T (2016) Size-controlled sub-micrometer spheroidized ZnO particles synthesis via plasma-induced processing in microdroplets. Mater Lett 166:81–84. https://doi.org/10.1016/j.matlet.2015.12.043
Arita S, Aoyagi N, Ohshita K et al (2017) Synthesis and characterization of spherical alumina nanoparticles by spray pyrolysis using radiofrequency plasma. J Ceram Soc JAPAN 125:539–542. https://doi.org/10.2109/jcersj2.16320
Gao Y, Masuda Y, Ohta H, Koumoto K (2004) Room-Temperature Preparation of ZrO2 Precursor Thin Film in an aqueous peroxozirconium-complex solution. Chem Mater 16:2615–2622. https://doi.org/10.1021/cm049771i
Izumi K, Murakami M, Deguchi T et al (1989) Zirconia Coating on Stainless Steel Sheets from Organozirconium compounds. J Am Ceramic Soc 72:1465–1468. https://doi.org/10.1111/j.1151-2916.1989.tb07677.x
Jo S, Raj R (2020) Transition to electronic conduction at the onset of flash in cubic zirconia. Scripta Mater 174:29–32. https://doi.org/10.1016/j.scriptamat.2019.07.043
Akasaka S, Amamoto Y, Yuji H, Kanno I (2021) Limiting current type yttria-stabilized zirconia thin-film oxygen sensor with spiral Ta2O5 gas diffusion layer. Sens Actuators B 327:128932. https://doi.org/10.1016/j.snb.2020.128932
Zhang S-C, Messing GL, Borden M (1990) Synthesis of solid, spherical Zirconia particles by Spray Pyrolysis. J Am Ceramic Soc 73:61–67. https://doi.org/10.1111/j.1151-2916.1990.tb05091.x
Manivasakan P, Karthik A, Rajendran V (2013) Mass production of Al2O3 and ZrO2 nanoparticles by hot-air spray pyrolysis. Powder Technol 234:84–90. https://doi.org/10.1016/j.powtec.2012.08.028
Nimmo W, Hind D, Ali NJ et al (2002) The production of ultrafine zirconium oxide powders by spray pyrolysis. J Mater Sci 37:3381–3387. https://doi.org/10.1023/A:1016549325319
Su Y-M, Kuo Y-L, Lin C-M, Lee S-F (2014) One-step fabrication of tetragonal ZrO2 particles by atmospheric pressure plasma jet. Powder Technol 267:74–79. https://doi.org/10.1016/j.powtec.2014.07.004
Verkouteren RM, Verkouteren JR (2011) Inkjet Metrology II: resolved effects of ejection frequency, fluidic pressure, and Droplet Number on Reproducible Drop-on-demand dispensing. Langmuir 27:9644–9653. https://doi.org/10.1021/la201728f
Dobre M, Bolle L (2002) Practical design of ultrasonic spray devices: experimental testing of several atomizer geometries. Exp Thermal Fluid Sci 26:205–211. https://doi.org/10.1016/S0894-1777(02)00128-0
Avvaru B, Patil MN, Gogate PR, Pandit AB (2006) Ultrasonic atomization: Effect of liquid phase properties. Ultrasonics 44:146–158. https://doi.org/10.1016/j.ultras.2005.09.003
Nitta K, Shimizu Y, Terashima K, Ito T (2021) Plasma-assisted synthesis of size-controlled monodisperse submicron gold particles using inkjet droplets. J Phys D: Appl Phys 54:33LT01. https://doi.org/10.1088/1361-6463/ac02f8
Yang J, Katagiri D, Mao S et al (2015) Generation of controlled monodisperse porous polymer particles by dipped inkjet injection. RSC Adv 5:7297–7303. https://doi.org/10.1039/C4RA13275K
Nitta K, Muneoka H, Shimizu Y et al (2023) Evaporation behavior of liquid microdroplets in atmospheric-pressure nonequilibrium plasma. Plasma Sources Sci Technol 32:055008. https://doi.org/10.1088/1361-6595/acd3ab
Nayak G, Simeni Simeni M, Rosato J et al (2020) Characterization of an RF-driven argon plasma at atmospheric pressure using broadband absorption and optical emission spectroscopy. J Appl Phys 128:243302. https://doi.org/10.1063/5.0035488
Maguire PD, Mahony CMO, Kelsey CP et al (2015) Controlled microdroplet transport in an atmospheric pressure microplasma. Appl Phys Lett 106:224101. https://doi.org/10.1063/1.4922034
Tsumaki M, Ito T (2017) Optical emission spectroscopy of atmospheric-pressure non-equilibrium plasma with mist injection. AIP Adv 7:125211. https://doi.org/10.1063/1.5011076
Bruggeman P, Iza F, Guns P et al (2010) Electronic quenching of OH(A) by water in atmospheric pressure plasmas and its influence on the gas temperature determination by OH(A – X) emission. Plasma Sources Sci Technol 19:015016. https://doi.org/10.1088/0963-0252/19/1/015016
Bruggeman P, Schram DC, Kong MG, Leys C (2009) Is the rotational temperature of OH(A–X) for discharges in and in contact with liquids a Good Diagnostic for determining the gas temperature? Plasma Processes Polym 6:751–762. https://doi.org/10.1002/ppap.200950014
Darwiche S, Nikravech M, Awamat S et al (2007) Optical emission spectroscopic investigation of hydrogen plasma used for modification of electrical properties of multi-crystalline silicon. J Phys D: Appl Phys 40:1030–1036. https://doi.org/10.1088/0022-3727/40/4/017
Sarani A, Nikiforov AYu, Leys C (2010) Atmospheric pressure plasma jet in ar and Ar/H2O mixtures: optical emission spectroscopy and temperature measurements. Phys Plasmas 17:063504. https://doi.org/10.1063/1.3439685
Cullen PJ, Milosavljevi V (2015) Spectroscopic characterization of a radio-frequency argon plasma jet discharge in ambient air. Progress of Theoretical and Experimental Physics 2015:63J01–63J00. https://doi.org/10.1093/ptep/ptv070
Sharma MK, Saikia BK, Phukan A, Ganguli B (2006) Plasma nitriding of austenitic stainless steel in N2 and N2–H2 Dc pulsed discharge. Surf Coat Technol 201:2407–2413. https://doi.org/10.1016/j.surfcoat.2006.04.006
Heywood H (1937) Numerical definitions of particle size and shape. Soc Chem Ind Symp Aggregates 56:149–154. https://doi.org/10.1002/jctb.5000560702
Cox EP (1927) A method of assigning numerical and percentage values to the degree of roundness of sand grains. J Paleontol 1:179–183
Blott SJ, Pye K (2008) Particle shape: a review and new methods of characterization and classification. Sedimentology 55:31–63. https://doi.org/10.1111/j.1365-3091.2007.00892.x
Zhao X, Vanderbilt D (2002) Phonons and lattice dielectric properties of zirconia. Phys Rev B 65:075105. https://doi.org/10.1103/PhysRevB.65.075105
Amiri A, Ingram GD, Maynard NE et al (2015) An Unreacted shrinking Core Model for Calcination and similar solid-to-gas reactions. Chem Eng Commun 202:1161–1175. https://doi.org/10.1080/00986445.2014.910771
Mezhericher M, Levy A, Borde I (2008) Modelling of particle breakage during drying. Chem Eng Process 47:1404–1411. https://doi.org/10.1016/j.cep.2007.06.018
Chen L, Mashimo T, Omurzak E et al (2011) Pure Tetragonal ZrO2 nanoparticles synthesized by Pulsed plasma in Liquid. J Phys Chem C 115:9370–9375. https://doi.org/10.1021/jp111367k
Maguire P, Rutherford D, Macias-Montero M et al (2017) Continuous In-Flight synthesis for On-Demand delivery of Ligand-Free Colloidal Gold Nanoparticles. Nano Lett 17:1336–1343. https://doi.org/10.1021/acs.nanolett.6b03440
Acknowledgements
Not Applicable.
Funding
This work was partially supported by JSPS KAKENHI (Grant Nos. 16H05988 and 19H01885). One of the authors (KN) was supported by a Grant-in-Aid from the JSPS Research Fellowship (Grant No. 20J21827).
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
KN: Conceptualization, Formal analysis, Investigation, Methodology, Data Curation, Funding acquisition, Writing—Original Draft. TS: Conceptualization, Formal analysis, Investigation, Methodology, Data Curation, Writing—Review & Editing. HM: Conceptualization, Methodology, Writing—Review & Editing. YS: Conceptualization, Resources, Writing—Review & Editing. HK: Conceptualization, Methodology, Writing—Review & Editing. KT: Conceptualization, Methodology, Project administration, Writing—Review & Editing. TI: Conceptualization, Methodology, Funding acquisition, Project administration, Supervision, Writing—Review & Editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nitta, K., Sakai, T., Muneoka, H. et al. Highly Reproducible Synthesis of Hollow Zirconia Particles via Atmospheric-Pressure Plasma Processing with Inkjet Droplets. Plasma Chem Plasma Process 44, 289–303 (2024). https://doi.org/10.1007/s11090-023-10412-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10412-0