Abstract
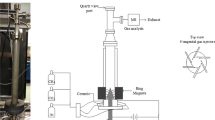
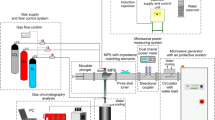
In this study, a technique of combining steam reforming with partial oxidation of CO2-containing natural gas in a gliding arc discharge plasma was investigated. The effects of several operating parameters including: hydrocarbons (HCs)/O2 feed molar ratio; input voltage; input frequency; and electrode gap distance; on reactant conversions, product selectivities and yields, and power consumptions were examined. The results showed an increase in either methane (CH4) conversion or synthesis gas yield with increasing input voltage and electrode gap distance, whereas the opposite trends were observed with increasing HCs/O2 feed molar ratio and input frequency. The optimum conditions were found at a HCs/O2 feed molar ratio of 2/1, an input voltage of 14.5 kV, an input frequency of 300 Hz, and an electrode gap distance of 6 mm, providing high CH4 and O2 conversions with high synthesis gas selectivity and relatively low power consumptions, as compared with the other processes (sole natural gas reforming, natural gas reforming with steam, and combined natural gas reforming with partial oxidation).





Similar content being viewed by others
References
Yang SW, Kondo JN, Hayashi K, Hirono M, Domen K, Hosono H (2004) Partial oxidation of methane to syngas over promoted C12A7. Appl Catal A 227:239–246
Bruke NR, Trimm DL (2006) Co-generation of energy and synthesis gas by partial oxidation of methane. Catal Today 117:248–252
Vasant RC, Kartick CM (2006) CO2 reforming of methane combined with steam reforming or partial oxidation of methane to syngas over NdCoO3 perovskite-type mixed metal-oxide catalyst. Appl Energy 83:1024–1032
Yu W, Ohmori T, Kataoka S, Yamamoto T, Endo A, Nakaiwa M, Itoh N (2008) A comparative simulation study of methane steam reforming in a porous ceramic membrane reactor using nitrogen and steam as sweep gases. Int J Hydrogen Energy 33:685–692
Ryi S, Park J, Kim D, Kim T, Kim S (2009) Methane steam reforming with a novel catalytic nickel membrane for effective hydrogen production. J Membr Sci 339:189–194
Santos CRM, Laureanny M, Fabio BP (2010) The effect of the addition of Y2O3 to Ni/α-Al2O3 catalysts on the autothermal. Catal Today 149:401–406
Rueangjitt N, Akarawitoo C, Sreethawong T, Chavadej S (2007) Reforming of CO2-containing natural gas using an AC gliding arc system: effect of gas component in natural gas. Plasma Chem Plasma Process 27:559–576
Sreethawong T, Thakonpatthanakun P, Chavadej S (2007) Partial oxidation of methane with air for synthesis gas production in a multistage gliding arc discharge system. Int J Hydrogen Energy 32:1067–1079
Anderson JA, Garcia MF (2005) Supported metals in catalysis. Imperial College Press, London
Rueangjitt N, Sreethawong T, Chavadej S (2008) Reforming of CO2-containing natural gas using an AC gliding arc system: effects of operational parameters and oxygen addition in feed. Plasma Chem Plasma Process 28:49–67
Wang B, Zhang X, Liu Y, Xu G (2009) Conversion of CH4, steam and O2 to syngas and hydrocarbons via dielectric barrier discharge. J Nat Gas Chem 18:94–97
Sobacchi MG, Saveliev AV, Fridman AA, Kennedy LA, Ahmed S, Krause T (2002) Experimental assessment of combined plasma/catalytic system for hydrogen production via partial oxidation of hydrocarbon fuels. Int J Hydrogen Energy 27:635–642
He JX, Han YY, Gao AH, Zhou YS, Lu ZG (2004) Investigation on methane decomposition and the formation of C2 hydrocarbons in DC discharge plasma by emission spectroscopy. Chin J Chem Eng 12:149–151
Nozaki T, Hattori A, Okazaki K (2004) Partial oxidation of methane using a microscale non-equilibrium plasma reactor. Catal Today 98:607–616
Kalra CS, Gutsol AF, Fridman AA (2005) Gliding arc discharge as a source of intermediate plasma for methane partial oxidation. IEEE Trans Plasma Sci 33:32–34
Paulmier T, Fulcheri L (2005) Use of non-thermal plasma for hydrocarbons reforming. Chem Eng J 106:59–71
Wang Y, Liu CJ, Zhang YP (2005) Plasma methane conversion in the presence of dimethyl ether using dielectric-barrier discharge. Energy Fuels 19:877–881
El Ahmar E, Met C, Aubry O, Khacef A, Cormier JM (2006) Hydrogen enrichment of a methane-air mixture by atmospheric pressure plasma for vehicle applications. Chem Eng J 116:13–18
Petitpas G, Rollier J-D, Darmon A, Gonzalez-Aguilar J, Matkemeijer R, Fulcheri L (2007) A comparative study of non-thermal plasma assisted reforming technologies. Int J Hydrogen Energy 32:2848–2867
Yang YC, Lee BJ, Chun YN (2009) Characteristics of methane reforming using gliding arc reactor. Energy 34:172–177
Liu C, Marafee A, Hill B, Xu G, Mallinson R, Lobban L (1996) Oxidative coupling of methane with ac and dc corona discharges. Ind Eng Chem Res 35:3295–3301
Rueangjitt N, Jittiang W, Pornmai K, Chamnanmanoontham J, Sreethawong T, Chavadej S (2009) Combined reforming and partial oxidation of CO2-containing natural gas using an AC multistage gliding arc system: effect of number of plasma reactors. Plasma Chem Plasma Process 29:433–453
Supat K, Chavadej S, Lobban LL, Mallinson R (2003) Combined steam reforming and partial oxidation of methane to synthesis gas under electrical discharge. Ind Eng Chem Res 42:1654–1661
Pornmai K (2012) Synthesis gas production from CO2-containing natural gas by combined steam reforming and partial oxidation in multi-stage gliding arc discharge system. Ph.D. dissertation, The Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, Thailand
Zielinski T, Kijenski J (2005) Plasma carbon black the new active additive for plastic. Compos A Appl Sci Manuf 36:467–471
Opalinska T, Zielinski T, Schmidt-Szalowski K (2003) Carbon black generation in gliding arc discharges. Pol J Chem 77:1357–1361
Supat K, Kruapong A, Chavadej S, Lobban LL, Millinson GR (2003) Synthesis gas production from partial oxidation of methane with air in AC electric gas discharge. Energy Fuels 17:471–481
Acknowledgements
The authors would like to thank Center of Excellence on Petrochemical and Materials Technology, Chulalongkorn University, Thailand for providing a Ph.D. scholarship for the first author and the Ratchadapisek Somphot Endowment Fund, Chulalongkorn University, Thailand for providing a financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pornmai, K., Jindanin, A., Sekiguchi, H. et al. Synthesis Gas Production from CO2-Containing Natural Gas by Combined Steam Reforming and Partial Oxidation in an AC Gliding Arc Discharge. Plasma Chem Plasma Process 32, 723–742 (2012). https://doi.org/10.1007/s11090-012-9371-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-012-9371-2