Abstract
We aimed to investigate whether the consumption of Egg White Hydrolysate (EWH) acts on nervous system disorders induced by exposure to Cadmium (Cd) in rats. Male Wistar rats were divided into (a) Control (Ct): H2O by gavage for 28 days + H2O (i.p. − 15th − 28th day); (b) Cadmium (Cd): H2O by gavage + CdCl2 − 1 mg/kg/day (i.p. − 15th − 28th day); (c) EWH 14d: EWH 1 g/kg/day by gavage for 14 days + H2O (i.p.- 15th − 28th day); (d) Cd + EWH cotreatment (Cd + EWHco): CdCl2 + EWH for 14 days; (e) EWH 28d: EWH for 28 days; (f) EWHpre + Cd: EWH (1st − 28th day) + CdCl2 (15th − 28th day). At the beginning and the end of treatment, neuromotor performance (Neurological Deficit Scale); motor function (Rota-Rod test); ability to move and explore (Open Field test); thermal sensitivity (Hot Plate test); and state of anxiety (Elevated Maze test) were tested. The antioxidant status in the cerebral cortex and the striatum were biochemically analyzed. Cd induces anxiety, and neuromotor, and thermal sensitivity deficits. EWH consumption prevented anxiety, neuromotor deficits, and alterations in thermal sensitivity, avoiding neuromotor deficits both when the administration was performed before or during Cd exposure. Both modes of administration reduced the levels of reactive species, and the lipid peroxidation increased by Cd and improved the striatum’s antioxidant capacity. Pretreatment proved to be beneficial in preventing the reduction of SOD activity in the cortex. EWH could be used as a functional food with antioxidant properties capable of preventing neurological damage induced by Cd.
Highlights
EWH prevented neurological damage induced by Cd exposure.
EWH prevented anxiety and neuromotor, and thermal sensitivity deficits before or during Cd exposure.
EWH improved the redox balance before or during Cd exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a heavy metal, without a highly toxic biological role defined and widely used by humans in industrial activities [1]. Its toxic effects have been demonstrated mostly in the cardiovascular [2] and the reproductive system [3]. The toxic damages caused by this metal are related to the increase of oxidative stress [4, 5], apoptosis [6, 7], autophagy [8], genetic deregulation [9, 10] and interaction with other elements such as calcium and zinc [11].
Cd can cross the blood-brain barrier (BBB) [12] and diffuse in the brain, increasing the oxidative stress and reducing antioxidant defenses [13], promoting behavioral changes [14, 15], alterations in cholinergic mechanics [16] and central and peripheral electrophysiological activity [17]. Cd poisoning has been related to amyotrophic lateral sclerosis development due to a deficiency of the brain SOD enzyme [18], increased risk of stroke [19], and neurodegenerative diseases [20].
The increase in contamination by this toxic metal has motivated research investigating the therapeutic effects of chelating and/or antioxidant compounds capable of protecting biological systems against their toxic damage [21]. Thus, functional foods such as egg white hydrolysate (EWH) have demonstrated multiple biological effects against Cd [22, 23] and other metal poisonings (Hg and Al) [24, 25]. Enzymatic hydrolysis to produce EWH results in an ingredient rich in bioactive peptides. Some beneficial effects have been reported, such as vasodilator [26], antihypertensive [27, 28], anti-inflammatory [29], angiotensin-converting enzyme inhibitor [27], and antioxidant [30,31,32] in different experimental models. Moreover, the EWH improved memory parameters and cognitive dysfunction of rats exposed to low doses of mercury and long-term exposure to Al, respectively [24, 32].
Herein, we investigated the protective effect of EWH against toxicity induced by high Cd concentrations in the nervous system of rats.
Methods
Animals and Experimental Design
Ninety-day male Wistar rats (290–330 g) were maintained at standard conditions (constant room temperature, humidity, and 12:12 h light-dark) with water and fed ad libitum in the Federal University of Pampa vivarium. The experimental protocols were performed according to the guidelines stated by the Brazilian Societies of Experimental Biology and National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH, 1996) and the Local Institution Animal Care and Use Committee (protocol number 012/2019 and 013/2019).
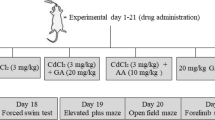
Animals were randomly distributed into six groups (Fig. 1):
-
1.
Control (Ct): drinking water by gavage + distilled water intraperitoneally (i.p.) for 28 days;
-
2.
Cadmium (Cd): Cadmium chloride – CdCl2 − 1 mg/kg i.p. for 14 days [33] + drinking water by gavage;
-
3.
EWH 14d: EWH 1 g/kg/day by gavage for 14 days + distilled water i.p [22]. ;
-
4.
Cd + EWHco: cotreatment with CdCl2 1 mg/kg i.p. + EWH 1 g/kg/day by gavage for 14 days [22];
-
5.
EWH 28d: EWH 1 g/kg/day by gavage for 28 days + distilled water i.p.;
-
6.
EWHpre + Cd: pretreatment with EWH 1 g/kg/day by gavage for 14 days + distilled water i.p. followed by CdCl2 − 1 mg/kg i.p. + EWH − 1 g/kg/day for 14 days.
The animals were submitted to all behavioral tests on days 13 and 29 of the protocol following the sequence: Open Field (OF), Neurologic Disabilities Scale (NDS), Rota-Rod (RR), Elevated Plus Maze (EPM) and Hot Plate (HP) (Fig. 1). This sequence was based on carrying out tests from lower capacity to generate stress to greater capacity to generate stress.
EWH Preparation
The egg white hydrolysate (EWH) was prepared for the treatment according to Garcés-Rimón et al. [29]. In summary, for obtained the EWH, the hydrolysis processes used Pepsin from pork stomach (E.C. 3.4.23.1 - Cardif, United Kingdom: 1:3000; E:S: 2:100 w-w, pH 2.0, 38 °C) mixed with commercial pasteurized egg white for 8 h. The inactivation of the enzyme was achieved by increasing the pH to 7.0 with 5 N NaOH. The EWH was centrifuged (2500 g, 15 min.), and the supernatants were frozen and lyophilized. The main bioactive peptides identified in EWH were previously checked by reverse-phase liquid chromatography-mass spectrometry (RP-HPLC-MS/MS): FRADHPFL, RADHPFL, YAEERYPIL, YRGGLEPINF, ESIINF, RDILNQ, IVF, YQIGL, SALAM, FSL [30, 34].
Behavior Tests
We evaluated the impact of Cd administration and EWH supplementation on neuromotor performance, locomotor and exploratory activity, motor coordination and balance, anxious-like behavior, and sensitivity. We performed all tests on days 13 and 29, equivalent to a pre and post-treatment evaluation (Fig. 1).
Experimental design. All rats were submitted to the Open Field (OF), Neurologic Disabilities Scale (NDS), Rota-rod (RR), Elevated Plus Maze (EPM) and Hot Plate (HP) and tests on days 13 and 29 of the protocol, equivalent to the initial and final times of Cd injection. The groups are represented in horizontal bars
Neurologic Disabilities Scale (NDS)
The Neurologic Disabilities Scale (NDS), to assess neuromotor performance, was performed. The test evaluates the animal’s ability to walk on the contralateral forelimb’s beam and flexion, retraction of the contralateral hindlimb, spontaneous spins, and bilateral seizures of the front legs. The animals were allocated and received a score ranging from 0 to 4, where 4 indicates severe neurological deficit, according to Bederson et al. [35].
Rota-Rod (RR)
To evaluate motor coordination and balance, we used the Rota-Rod (RR) task. The apparatus consisted of a cylinder; suspended 20 cm from the device surface, driven by a gear that maintains a constant speed. First, we did habituation to the apparatus at a rate of 16 rotations per minute (rpm). After, we performed the test at 26 rpm. We placed the rats on the cylinder, and in 5 min, we recorded: (i) the time for the first fall and (ii) the number of falls [36].
Open Field (OF)
We assessed the exploratory and locomotor activity by open field (OF) task, as previously described by Bonini et al. [37]. The apparatus consists of a box made of wood a box (50 × 50 × 50 cm) in which black lines divide the floor into 12 equal squares. We placed the rats individually in the center of the apparatus, and during a session of five minutes, we recorded the number of crossings and rearings.
Hot Plate (HP)
To analyze the sensitivity/pain threshold of the rats, we used an HP test. The apparatus consists of a plate enclosed by a 25 cm high, lidded perspex box measuring 145 × 275 mm. The test consisted of placing a rat on a metal platform. This platform is progressively heated. We recorded the time that the rats led to withdrawing their paws. To avoid injuries in the paws, we imposed a ceiling of 60 s [38].
Elevated plus Maze (EPM)
The Elevated Plus Maze test (EPM) was used to evaluate the anxiety behavior; each rat was placed in the maze, which contains two open and two closed arms, for 5 min, and the time in the open arms of the maze was monitored for each rat as previously described [39].
Biochemicals Tests
At the end of treatments and behavior testing, rats were euthanized, and the cortex and striatum from the brain were quickly dissected and homogenized (50mM Tris HCl, pH 7.4, 1/10, w/v), centrifuged (2400 g, 10 min, 4 °C) and frozen at − 80 °C for performed oxidative stress biochemical analysis. Levels of reactive oxygen species (ROS) were determined by the spectrofluorometric method described by Loetchutinat et al. [40]. The ROS levels were expressed as fluorescence units (FU). Lipid peroxidation was measured as malondialdehyde (MDA) using a colorimetric method, as previously described by Ohkawa et al. [41], with modifications [42], and results were expressed as nmol of MDA/mg of protein. The total antioxidant capacity in the cortex and striatum tissue was measured by Ferric Reducing Antioxidant Power (FRAP) Assay [43], with modifications [42] and results were presented with particular reference to Trolox equivalents, Trolox (µmol/mL). Superoxide dismutase (SOD) activity was assayed using spectrophotometry, as described by Misra and Fridovich [44], and expressed as units (U)/mg of protein.
According to the Bradford method [45], the protein was measured using bovine serum albumin as a standard. All reagents and salts were obtained from Sigma-Aldrich and Merck (Darmstadt, Germany).
Statistical Analysis
Results are expressed as mean ± SD. Data from behavior tests were analyzed using two-way ANOVA to assess the effects of different treatments over time (time factor) and to assess the effects of different treatments between groups (treatment factor), as well as the interaction between the two factors (time x treatment factors). Data from biochemical tests were analyzed using two-way ANOVA to assess the effects of Cadmium administration (Cd factor) and to assess the effects of EWH between groups (treatment factor), as well as the interaction between the two factors (Cd x EWH factors). When two-way ANOVA showed a statistically significant difference in any of these factors, or interaction between them, a Bonferroni’s post-hoc analysis was performed. Differences were considered statistically significant when P < 0.05.
Results
Behavioral Tests
Neurological Disabilities Scale (NDS)
In the NDS, two-way ANOVA revealed an effect over time (F (1, 52) = 6.834, P = 0.0117) and an effect of the treatment (F (5, 52) = 8.185, P < 0.001). There was an interaction between both factors (F (5,52) = 8.185, P = 0.0001).
Post-hoc analyses showed that the neuromotor performance did not alter over time in the Ct group (t (52) = 0.000, P > 0.999 for 0 vs. 14th day, Table 1). On the other hand, cadmium administration for 14 days induced a neurological deficit (t (52) = 7.466, P < 0.0001, Table 1). EWH supplementation for 14 days did not affect neurological performance over time (t (52) = 0.000, P > 0.999 for the EWH14d group). In the same way, EWH administration for 28 days did not induce the neurological deficit (t (52) = 0.000, P > 0.999 for EWH28d, Table 1). Both Cd + EWHco (t = 0.519, P > 0.9999, Table 1) and EWHpre + Cd (t = 0.000, P > 0.999, Table 1) groups did not show neurological deficits over time.
When we compare groups, animals from all groups did not express neurological deficit on day 0 (P > 0.05 for all groups, Table 1). On the 14th day, animals from the Cd group showed a neurological deficit compared to the Ct group (t (104) = 6.816, P < 0.0001, Table 1). Animals from both groups Cd + EWHco (t (104) = 6.254, P < 0.0001) and EWHpre + Cd (t (104) = 6.605, P < 0.0001) did not show neurological deficit compared to the Cd group.
Rota-Rod (RR) Task
On the RR test, two-way ANOVA did not reveal an effect on the number of falls over time (F (1, 49) = 0.242, P = 0.622) but revealed an effect of the treatment (F (5, 49) = 2.673, P < 0.032).
There was no change in the number of falls over time (P > 0.05 for all groups, Fig. 2A). In the same way, there was no change in the number of falls when comparing the groups on day 0 (P > 0.05 for all groups, Fig. 2A). On the 14th day, the Cd group showed no change in the number of falls compared to the Ct group (t (98) = 0.9668, P > 0.999, Fig. 2A). However, there was a reduction in the number of falls in animals in the Cd + EWHco group compared to animals in the Cd group (t (98) = 3.095, P = 0.0384, Fig. 2A).
Regarding the latency for the first fall, two-way ANOVA revealed no effect over time (F (1, 48) = 1.086, P = 0.3026), nor effect of the treatment between the groups (F (5, 48) = 1.508, P = 0.2049). There was no interaction between factors (F (5, 48) = 0.7308, P = 0.6038) (Fig. 2B).
Effects of EWH on motor coordination and balance evaluated by Rota-Rod (RR) in Cd-exposure rats. (A) The number of falls. (B) Latency to the first fall, in seconds. Data are presented as the mean ± SD. #P < 0.05 in the groups’ comparison (n = 5–13). Ct: drinking water by gavage + distilled water intraperitoneally (i.p.) for 28 days; Cd: Cadmium chloride – CdCl2 − 1 mg/kg i.p. for 14 days + drinking water by gavage; EWH 14d: egg white hydrolysate 1 g/kg/day by gavage for 14 days + distilled water i.p.; Cd + EWHco: cotreatment with CdCl2 1 mg/kg i.p. + EWH 1 g/kg/day by gavage for 14 days; EWH 28d: egg white hydrolysate 1 g/kg/day by gavage for 28 days + distilled water i.p.; EWHpre + Cd: pretreatment with EWH 1 g/kg/day by gavage for 14 days + distilled water i.p. followed by CdCl2 − 1 mg/kg i.p. + EWH − 1 g/kg/day for 14 days
Open Field (OF)
On the OF, two-way ANOVA revealed an effect on the crossing over time (F (1, 52) = 51.56, P < 0.0001) and no effect of the treatment (F (5, 52) = 4.097, P = 0.0033). There was no interaction between factors (F (5, 52) = 1.704, P = 0.1501).
Animals from the Ct group showed no change in locomotor activity over time (t (52) = 1.563, P = 0.7449; Fig. 3A). However, administration of Cd for 14 days led to a reduction in locomotor activity over time (t (52) = 4.662, P = 0.0001; Fig. 3A). Surprisingly, the supplementation with EWH for 14 days reduced locomotor activity (t (52) = 4.086, P = 0.0009; Fig. 3A). Cotreatment with EWH for 14 days and pre-administration for 28 days were not able to prevent the impairment of locomotor activity induced by Cd (t (52) = 4.842, P < 0.0001 for Cd + EWHco; t (52) = 2.789, P = 0.0443 for EWHpre + Cd; Fig. 3A). EWH supplementation for 28 days did not affect locomotor activity per se (t (52) = 0.9823, P > 0.999 for EWH28d; Fig. 3A).
There was no statistically significant difference between the groups in the number of crossing days 0 or 14 (P > 0.05 for all groups; Fig. 3A).
We also assessed exploratory activity by counting the number of rearing. Two-way ANOVA revealed an effect over time (F (1, 52) = 41.73, P = 0.0001) but not an effect of the treatment (F (5, 52) = 2.092, P = 0.0815). There was no interaction between factors (F (5, 52) = 0.7412, P = 0.5962).
In the control condition, there was no change in exploratory activity over time (t (51) = 1.681, P = 0.593; Fig. 3B). Animals from the Cd groups showed a reduction in exploratory activity (t (51) = 3.613, P = 0.004; Fig. 3B). Supplementation with EWH for 14 days also reduced exploratory activity (t (51) = 3.525, P = 0.0054; Fig. 3B). We observed similar results in both groups Cd + EWHco (t (51) = 3.525, P = 0.024; Fig. 3B) and EWHpre + Cd (t (51) = 3.716; P = 0.003; Fig. 3B). EWH supplementation for 28 days did not affect locomotor activity (t (51) = 1.159, P > 0.999; Fig. 3B). There was no statistically significant difference between the groups in the number of rearing on days 0 and 14 (P > 0.05 for all groups; Fig. 3B).
Effects of Egg White Hydrolysate (EWH) on locomotor and exploratory activities evaluated by Open Field (OF) in Cd-exposure rats. (A) The number of crossings. (B) The number of rearings. Data are presented as the mean ± SD. *P < 0.05 vs. day 0 (n = 5–13). Ct: drinking water by gavage + distilled water intraperitoneally (i.p.) for 28 days; Cd: Cadmium chloride – CdCl2 − 1 mg/kg i.p. for 14 days + drinking water by gavage; EWH 14d: egg white hydrolysate 1 g/kg/day by gavage for 14 days + distilled water i.p.; Cd + EWHco: cotreatment with CdCl2 1 mg/kg i.p. + EWH 1 g/kg/day by gavage for 14 days; EWH 28d: egg white hydrolysate 1 g/kg/day by gavage for 28 days + distilled water i.p.; EWHpre + Cd: pretreatment with EWH 1 g/kg/day by gavage for 14 days + distilled water i.p. followed by CdCl2 − 1 mg/kg i.p. + EWH − 1 g/kg/day for 14 days
Hot Plate (HP)
On the HP test, two-way ANOVA revealed an effect over time (F (1, 52) = 4.532, P = 0.038) but not an effect of the treatment (F (5, 52) = 1.699, P = 0.151). There was an interaction between two factors (F (5, 52) = 2.577, P = 0.0371).
Ct group rats showed no change in peripheral thermal sensitivity over time (t (52) = 0.5368, P > 0.999, Fig. 4). Cd administration increased the latency in the withdrawal of the paws (t (52) = 3.970, P = 0.001, Fig. 4). Animals from both groups Cd + EWHco (t (52) = 0.134, P > 0.999, Fig. 4) and EWHpre + Cd (t (52) = 1.839, P = 0.429, Fig. 4) did not show alteration in the latency of withdrawal of the paws over time.
When comparing the groups, animals from all groups did not express alteration in the latency of withdrawal of the paws on day 0 (P > 0.05 for all groups, Fig. 4). On the 14th day, animals from the Cd group did not demonstrate increased paws’ withdrawal compared with the Ct group (t (104) = 0.2720, P < 0.090, Fig. 4). Animals from the EWH14d group showed a decrease in the latency of the paws’ withdrawal compared with the Cd group (t (104) = 0.5648, P = 0.0033, Fig. 4). In the same way, animals from the Cd + EWHco showed a decrease in the latency of the paws’ withdrawal compared with the Cd group (t (104) = 0.7113, P = 0.0378).
Effects of Egg White Hydrolysate (EWH) on peripherical thermal sensibility evaluated by Hot Plate (HP) in Cd-exposure rats (latency to paw withdraw). Data are presented as the mean ± SD. *P < 0.05 vs. day 0; #P < 0.05 for comparison between groups (n = 5–14). Ct: drinking water by gavage + distilled water intraperitoneally (i.p.) for 28 days; Cd: Cadmium chloride – CdCl2 − 1 mg/kg i.p. for 14 days + drinking water by gavage; EWH 14d: egg white hydrolysate 1 g/kg/day by gavage for 14 days + distilled water i.p.; Cd + EWHco: cotreatment with CdCl2 1 mg/kg i.p. + EWH 1 g/kg/day by gavage for 14 days; EWH 28d: egg white hydrolysate 1 g/kg/day by gavage for 28 days + distilled water i.p.; EWHpre + Cd: pretreatment with EWH 1 g/kg/day by gavage for 14 days + distilled water i.p. followed by CdCl2 − 1 mg/kg i.p. + EWH − 1 g/kg/day for 14 days
Elevated plus Maze (EPM)
Regarding entries in the close arms on the EPM test, two-way ANOVA revealed an effect over time (F (1, 52) = 17.65, P = 0.0001) and the effect of the treatment (F (5, 52) = 2.998, P = 0.0188). There was an interaction between two factors (F (5, 52) = 2.789, P = 0.0264).
The Ct group did not show a change in the number of entries in the closed arms over time (t (52) = 1.036, P > 0.999, Fig. 5A). Cd administration decreased the number of entries in the closed arms on the 14th day when we compared it to day 0 (t (52) = 5.450, P < 0.0001, Fig. 5A). The EWH for 14 days was not able to change the number of entries on the closed arms (t (52) = 1.036, P > 0.9999, Fig. 5A). However, the Cd + EWHco group showed a decrease in the number of entries in the closed arms (t (52) = 2.763, P > 0.0473, Fig. 5A). The EWH 28d group showed no change in the number of entries on the closed arms (t (52) = 0.000, P > 0.9999, Fig. 5A). Furthermore, the EWHpre + Cd (t (52) = 1.456, P = 0.9076, Fig. 5A) did not show alteration in the number of entries in the closed arms.
When we compared the groups, animals from all groups did not express alteration in the number of entries in the closed arms on day 0 (P > 0.05 for all groups, Fig. 5A). On the 14th day, animals from the Cd group demonstrated a decrease in the number of entries in the closed arms compared with the Ct group (t (104) = 0.1424, P = 0,0204, Fig. 5A). Animals from the EWH14d group showed no change in the number of entries in the closed arms compared with the Ct group (t (104) = 0.7180, P > 0.9999, Fig. 5A). The animals from the Cd + EWHco did not show a change in the number of entries in the closed arms compared with the Cd group (t (104) = 1.962, P = 0.7857, Fig. 5A). In the same way, the EWHpre + Cd group did not change in the number of entries in the closed arms when we compared it with the Cd group (t (104) = 1.031, P > 0.9999, Fig. 5A).
We assessed the number of entries in the opened arms on the EPM test, two-way ANOVA revealed an effect over time (F (1, 52) = 22.92, P < 0.0001) and the effect of the treatment (F (5, 52) = 3.595, P = 0.0072). However, there was no interaction between the two factors (F (5, 52) = 0.9619, P = 0.4497) (Fig. 5B).
The Ct group showed no change in the number of entries in the opened arms over time (t (52) = 1.400, P > 0.999, Fig. 5B). Cd administration decreased the number of entries in the opened arms on the 14th day when we compared it to day 0 (t (52) = 4.185, P < 0.0007, Fig. 5B). On the other hand, the EWH14d group (t (52) = 1.507, P = 0.8269, Fig. 5B), Cd + EWHco group (t (52) = 2.691, P = 0.0573, Fig. 5B), EWH 28d group (t (52) = 0.6090, P > 0.999, Fig. 5B), and EWHpre + Cd group (t (52) = 2.383, P = 0.1251, Fig. 5B) did not show a change in the number of entries in the opened arms over the time.
The post-hoc analyses did not show a statistically significant difference in the number of entries in the opened arms when we compared the groups on day 0 (P > 0.05 for all groups, Fig. 5B). Likewise, there was no statistically significant difference in the number of entries into the opened arms when we compared between groups on day 14th (P > 0.05 for all groups, Fig. 5B).
Regarding the time spent in the closed arms, two-way ANOVA revealed no effect over time (F (1, 51) = 1.771, P = 0.1892); however, there was an effect of the treatment (F (5, 52) = 3.595, P = 0.0072). There was no interaction between the two factors (F (5, 51) = 0.4633, P = 0.8017).
There was no statistically significant difference in the time spent in the closed arms on the 14th day when we compared it with day 0 (P > 0.05 for all groups, Fig. 5C) in the post-hoc test. Despite the ANOVA analysis has shown an effect of the treatment between the groups, the post-hoc test showed no statistically significant difference in the time spent in the closed arms neither on day 0 (P > 0.05 for all groups, Fig. 5C) nor on day 14th (P > 0.05 for all groups, Fig. 5C).
In addition, two-way ANOVA did not reveal an effect on the time spent in the open arms over time (F (1, 52) = 1.919, P = 0.1719); however, there was an effect of the treatment (F (5, 52) = 2.502, P = 0.0419). There was no interaction between the two factors (F (5, 51) = 0.5919, P = 0.7062).
There was no statistically significant difference in the time spent in the opened arms on the 14th day when we compared it with day 0 (P > 0.05 for all groups, Fig. 5D) in the post-hoc test. Despite the ANOVA analysis has shown an effect of the treatment between the groups, the post-hoc test showed no statistically significant difference in the time spent in the open arms neither on day 0 (P > 0.05 for all groups, Fig. 5C) nor on day 14th (P > 0.05 for all groups, Fig. 5D)
Effects of Egg White Hydrolysate (EWH) on anxiety levels by Elevate Plus Maze (EPM) in Cd-exposure rats. (A) Entries on closed arms. (B) Entries on open arms. (C) Time spent on closed arms. (D) Time spent on open arms. *P < 0.05 vs. day 0; #P < 0.05 in the groups’ comparison (n = 9). Ct: drinking water by gavage + distilled water intraperitoneally (i.p.) for 28 days; Cd: Cadmium chloride – CdCl2 − 1 mg/kg i.p. for 14 days + drinking water by gavage; EWH 14d: egg white hydrolysate 1 g/kg/day by gavage for 14 days + distilled water i.p.; Cd + EWHco: cotreatment with CdCl2 1 mg/kg i.p. + EWH 1 g/kg/day by gavage for 14 days; EWH 28d: egg white hydrolysate 1 g/kg/day by gavage for 28 days + distilled water i.p.; EWHpre + Cd: pretreatment with EWH 1 g/kg/day by gavage for 14 days + distilled water i.p. followed by CdCl2 − 1 mg/kg i.p. + EWH − 1 g/kg/day for 14 days
Biochemical Results
Biochemical analysis corroborates our behavioral findings. On ROS levels, two-way ANOVA revealed an effect of Cd (F (1, 31) = 43.40, P < 0.0001), an effect of the treatment with EWH (F (2, 31) = 4.559, P = 0.00184), and an interaction between factors (F (2, 31) = 11.06, P = 0.0002).
Animals exposed to Cd increased the ROS levels in the cortex (t (31) = 7.626, P < 0.0001; Fig. 6A) and striatum (t (31) = 6.555, P < 0.0001; Fig. 6a). However, EWH pretreatment (EWHpre + Cd) (cortex: t (31) = 5.400, P = 0.0001, Fig. 6A; striatum: t (31) = 4.417, P = 0.0017, Fig. 6a) and cotreatment (Cd + EWHco) (cortex: t (31) = 3.776, P = 0.0102, Fig. 6A; striatum: t (31) = 3.984, P = 0.0057, Fig. 6a) significantly reduced the ROS levels in both cerebral areas investigated. ROS levels in Cd + EWHco remain elevated when compared to the cortex of control rats (t (31) = 3.710, P = 0.0122; Fig. 6A). There was no statistically significant difference between Ct, EWH14d, and EWH28d groups in ROS levels in both cerebral areas investigated (P > 0.05 for all groups; Fig. 6A-a).
Lipid peroxidation, in the same way, shows an effect of Cd (F (1, 38) = 42.14, P < 0.0001), and an effect of the treatment with EWH (F (2, 38) = 9.926, P = 0.0003) and there was no interaction between the two factors (P = 0.45).
Cd exposure increase significantly MDA levels in the cortex and striatum (t (38) = 5.545, P < 0.0001, Fig. 6B; t (38) = 5.853, P < 0.0001, Fig. 6b). Both EWH treatments reversed the lipid peroxidation to control levels in the cortex (Cd + EWHco: t (38) = 3.697, P < 0.0103; EWHpre + Cd: F (1, 38) = 3.272, P < 0.0341) and striatum (Cd + EWHco: t (38) = 3.752, P < 0.0088; EWHpre + Cd: t (38) = 4.177, P < 0.0030). There was no statistically significant difference between Ct, EWH14d, and EWH28d groups in MDA levels in the cortex or the striatum (P > 0.05 for all groups; Fig. 6B-b).
The antioxidant capacity did not show an effect of Cd (F (1, 39) = 1.427, P = 0.23), and showed an effect of the treatment with EWH (F (2, 39) = 7.357, P = 0.0019) and an interaction between the two factors (F (2, 39) = 4.551, P = 0.0167).
The antioxidant capacity was reduced in the cortex (t (39) = 3.371, P = 0.0255, Fig. 6C) and striatum (t (39) = 5.161, P = 0.0001, Fig. 6C) on Cd exposure rats. EWH pretreatment (EWHpre + Cd: t (39) = 1.950, P = 0.0003, Fig. 6D); EWH treatments had the effect of restoring antioxidant capacity on striatum (EWHpre + Cd: t (39) = 8.429, P < 0.0001; Cd + EWHco: t (39) = 6.067, P < 0.0001, Fig. 6c). Control situations did not differ from each other in the cortex or striatum (P > 0.05 for all groups; Fig. 6C-c).
SOD activity also was evaluated, and two-way ANOVA revealed an effect of Cd (F (1, 30) = 43.44, P < 0.0001), and an effect of the treatment with EWH (F (2, 30) = 6.193, P = 0.0056) and an interaction between factors (F (2, 30) = 8.878, P = 0.0009).
The SOD activity did not differ between the control groups (P > 0.05). However, was reduced in Cd-exposure rats (t (30) = 5.494, P = 0.0001, Fig. 6d) independently of EWH treatment in the striatum (EWHpre + Cd: t (33) = 4.615, P = 0.0009; Cd + EWHco: t (33) = 3.963, P = 0.0056). However, in the cortex, the reduction of SOD activity observed in the Cd group (t (30) = 5.004, P = 0.0003) was restored only in EWH-pretreated rats (t (30) = 4.455, P = 0.0004) (Fig. 6d). There was no statistically significant difference between Ct, EWH14d, and EWH28d groups in SOD activity in both cerebral areas investigated (P > 0.05 for all groups; Fig. 6D-d).
Effect of Egg White Hydrolysate (EWH) on oxidative stress in Cd-exposure rats. (A and a) Reactive oxygen species (ROS) levels expressed in fluorescence units (FU). (B and b) lipoperoxidation by Thiobarbituric Acid Reactive Substances (TBARS) expressed as nmol MDA/mg protein. (C and c) Total antioxidant capacity by Ferric Reducing Antioxidant Power Assay (FRAP) expressed as Trolox (µmol/mL). (D and d) Superoxide dismutase activity as units (U)/mg of protein. Capital letters indicate the measurements in the cortex, and lower-case in the striatum. Data are presented as the mean ± SD. P < 0.05 * vs. Ct group; # vs. Cd group (ANOVA followed by Bonferroni post-hoc test) (n = 8). Ct: drinking water by gavage + distilled water intraperitoneally (i.p.) for 28 days; Cd: Cadmium chloride – CdCl2 − 1 mg/kg i.p. for 14 days + drinking water by gavage; EWH 14d: egg white hydrolysate 1 g/kg/day by gavage for 14 days + distilled water i.p.; Cd + EWHco: cotreatment with CdCl2 1 mg/kg i.p. + EWH 1 g/kg/day by gavage for 14 days; EWH 28d: egg white hydrolysate 1 g/kg/day by gavage for 28 days + distilled water i.p.; EWHpre + Cd: pretreatment with EWH 1 g/kg/day by gavage for 14 days + distilled water i.p. followed by CdCl2 − 1 mg/kg i.p. + EWH − 1 g/kg/day for 14 days
Discussion
In this work, we demonstrated that Cd exposure is associated with decreased neurological and locomotor function in rats and that the consumption of an EWH treated with pepsin can both prevent (pretreatment with EWH) and avoid (cotreatment with EWH) partially this damage.
Cd is a toxic metal since from low to high exposure doses [46]. In addition, it is considered a widespread environmental pollutant, and the main source of toxic exposure is particulate inhalation by cigarette smoke [47], but it is also present in small amounts in foods [48]. Due to its presence in several products consumed by the general population, studies that report its toxicity are being performed. Furthermore, the literature has well described that Cd produces behavioral disorders and that these changes are related to its neurotoxic effect [47].
According to our best knowledge, for the first time, we demonstrated the neuromotor deficits caused by Cd through NDS evaluation in rats. Cd exposure is mainly related to brain and liver damage, which regulate neurological activities and energy metabolism, respectively [49]. Some authors suggest that Cd may cross the BBB and accumulate in the brain, damaging the central nervous system [50, 51], which could help to explain the observed deficits. On the other hand, the rats pre or cotreated with EWH did not present alterations in NDS. Consumption of EWH also decreased the number of falls in the RR test, demonstrating an improvement in motor coordination and balance.
However, the administration of EWH was not able to prevent or revert all types of deficits caused by Cd. Regarding the locomotor and exploratory activity of rats, assessed through the OF, the results showed that Cd induces locomotor and exploratory deficits and that both preexposure and cotreatment with EWH cannot prevent these deficits. A recent study using a dose curve of 1, 2, and 3 mg/g of Cd administered intraperitoneally demonstrated that the higher dose of Cd generates greater locomotor damage assessed in the OF, corroborating our results on the neurotoxic role and suggesting dose-dependent activity of Cd [47]. Exposure to high Cd doses has been previously related to increased oxidative stress in the reproductive and cardiovascular systems [22, 52]. It has been also reported that the accumulation of Cd in the nervous system generates an increase in reactive species [53], mainly superoxide anion [54], as occurs in other organs. Haider et al. [47] related locomotor damage to increased levels of malondialdehyde (MDA), a secondary product of lipid oxidation, and decreased levels of superoxide dismutase (SOD), an important antioxidant defense in the CNS.
In our study, increased levels of ROS and lipid peroxidation in the brain and a reduction in antioxidant defense were also observed. According to our results, high levels of lipid peroxidation were induced in the brain tissue of mice exposed to a low dose of Cd (1 and 2 mg/kg in drinking water after 30 days), and these findings were related to damage to antioxidants defense [55]. Acute exposure to a single dose similar to the one used in our study (1 mg/kg i.p.) also increased lipid peroxidation in the brain [56]. Chronic exposure to low concentrations of Cd (10, 25, and 50 mg/L in drinking water for 30 days) has also been reported to generate oxidant-antioxidant imbalance with damage at the GSH level and SOD, Catalase, and GPx activity [57]. In our findings, the scavenger capacity of EWH seems to have prevented the reduction of SOD activity by protecting neurological functions.
EWH, which has previously demonstrated neuroprotective properties associated with its antioxidant effect [24, 25], was used in our study with the intent to prevent or reverse motor deficits caused by Cd. However, although it was able to avoid the deficits measured by NDS and RR, we did not observe a reversal of the damage in the locomotor and exploratory activity in OF. These results partially agree with previous studies from our research group, which demonstrate that the administration of EWH does not alter the locomotor and exploratory capacity of animals that have been intoxicated with toxic metals, such as aluminum [25] or mercury [24].
Regarding the sensorial function, Cd-induced increase of the paw withdrawal on the HP task, which indicates deficits in the sensorial perception. Consumption of EWH was able to avoid this increase. A similar result has been recently described. Papp et al. [58] demonstrated that the somatosensory system was impaired after Cd exposure, even without detectable Cd deposition in the rat’s brain, and that these damages remained up to six weeks after exposure to Cd.
In the EPM task, we verified that Cd reduced the number of entries in the closed and open arms. Similarly, to our findings, behavioral damage induced by Cd-exposure has been reported at lower doses of exposure. Abdalla et al. [59] treated rats chronically with 2.5 mg/kg of Cd orally for 45 days and identified anxiolytic behavior in animals, relating this result to increased oxidative stress. Using the intraperitoneal route, Lamtai et al. [60] used a lower dose (0.25 and 0.5 mg/kg/day) and the same dose used in this study (1 mg/kg/day); however, twice of time of exposure (4 weeks), demonstrated that had lower entries in the open arms and exploratory behavior affected.
Different bioactive peptides derived from food proteins of different sources such as fish, soy, whey, milk, rice, silk, red algae, ginseng, and walnut have demonstrated neuroprotective capacity related to their anti-inflammatory, antioxidant, and/or anti-neurodegenerative properties, deserving to be investigated against neurological disorders [61]. However, there are currently very few studies that investigate the neuroprotective potential peptides derived from egg proteins, but some studies have shown promising results. A study carried out in healthy humans showed that dietary supplementation with an egg white lysozyme hydrolysate slightly improved the behavioral responses of the individuals. The authors associated this improvement with an increase in tryptophan levels and a consequent increase in the synthesis and release of serotonin in the CNS [62].
Egg white has naturally occurring low molecular weight (< 3 kDa) peptides with high antioxidant properties [63], and enzymatic hydrolysis of egg white proteins seems to strengthen this antioxidant effect, as observed in our study. Moreover, our EWH seems to have an essential benefit against metal intoxication. This functional food demonstrated an antioxidant effect against other metals reported by Rizzetti et al. [24], which prevented memory loss induced by exposure to low Hg concentration. EWH also prevented memory loss and cognitive deficits induced by exposure to low concentration (8.3 mg/kg for 60 days) and high concentration (100 mg/kg for 42 days) via drinking water from Al, as reported by Martinez et al. [25].
Conclusions
Our findings showed that exposure to high Cd concentrations causes severe neurological damage with reduced exploratory capacity, motor dysfunctions, sensibility reduction, and induction of anxiety; on the other hand, pre or co-administration EWH can reduce oxidative stress and prevent partially the neurological damage induced by the metal. In conclusion, EWH could be used as a functional food with antioxidant properties capable of preventing neurological damage induced by Cd.
Future Directions
Regarding the future development and commercialization of EWH as “functional food”, will be necessary to evaluate their bioactivity through intervention studies and clinical trials to ensure the effectiveness and safety of the product before its commercialization and more studies about bioavailability of isolated peptides and whole hydrolysates will be at the forefront of future functional food research. Another important aspect to highlight is that although there is growing interest in food-derived bioactive peptides, and it is relatively low cost to produce these peptides or their hydrolysates at laboratory scale, few researches aimed on the optimization process of food derived peptides and/or their hydrolysates, to obtain practical techniques used in food industry, making the leap from lab-scale to a commercial or pilot plant production because, in so many cases, it is too costly to produce them at industrial scale.
References
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Inter Tox 7:60–72. https://doi.org/10.2478/intox-2014-0009
Eum KD, Lee MS, Paek D (2008) Cadmium in blood and hypertension. Sci Total Environ 407:147–153. https://doi.org/10.1016/j.scitotenv.2008.08.037
Ren Y, Shao W, Zuo L, Zhao W, Qin H, Hua Y, Lu D, Mi C, Zeng S, Zu L (2019) Mechanism of cadmium poisoning on testicular injury in mice. Oncol lett 18:1035–1042. https://doi.org/10.3892/ol.2019.10418
Nemmiche S (2017) Oxidative signaling response to Cadmium exposure. Toxicol Sci 156:4–10. https://doi.org/10.1093/toxsci/kfw222
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. ToxicolAppl Pharmacol 238:209–214. https://doi.org/10.1016/j.taap.2009.01.029
Hamada T, Tanimoto A, Sasaguri Y (1997) Apoptosis induced by cadmium. Apoptosis 2:359–367. https://doi.org/10.1023/a:1026401506914
Aktas C, Kanter M, Erboga M, Ozturk S (2012) Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol Ind Health 28:122–130. https://doi.org/10.1177/0748233711407242
Messner B, Türkcan A, Ploner C, Laufer G, Bernhard D (2016) Cadmium overkill: autophagy, apoptosis and necrosis signalling in endothelial cells exposed to cadmium. CMLS 73:1699–1713. https://doi.org/10.1007/s00018-015-2094-9
Takahashi S, Yamamoto C, Kaji T (2014) Expression of ZIP8 in vascular endothelial cells after exposure to cádmium. Yakugaku Zasshi 134:805–807. https://doi.org/10.1248/yakushi.14-00017-6
Lee JY, Tokumoto M, Hattori Y, Fujiwara Y, Shimada A, Satoh M (2016) Different regulation of p53 expression by Cadmium exposure in kidney, liver, intestine, vasculature, and brain astrocytes. Toxicol Res 32:73–80. https://doi.org/10.5487/TR.2016.32.1.073
Costa -D, Botana R, Piñero D, Proverbio S, Marín F R (2016) Cadmium inhibits motility, activities of plasma membrane ca(2+)-ATPase and axonemal dynein-ATPase of human spermatozoa. Andrologia 48:464–469. https://doi.org/10.1111/and.12466
Shukla A, Shukla GS, Srimal RC (1996) Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol 15:400–405. https://doi.org/10.1177/096032719601500507
Branca J, Morucci G, Pacini A (2018) Cadmium-induced neurotoxicity: still much ado. Neural Reg Res 13:1879–1882. https://doi.org/10.4103/1673-5374.239434
Gupta A, Gupta A, Murthy RC, Ali MM, Chandra SV (1993) Neurobehavioral and neurochemical changes in growing rats after cadmium exposure. Toxicol Envron Chem 39:153–160. https://doi.org/10.1080/02772249309357912
Viaene MK, Masschelein R, Leenders J, De Groof M, Swerts LJ, Roels HA (2000) Neurobehavioural effects of occupational exposure to cadmium: a cross sectional epidemiological study. Occup Environ Med 57:19–27. https://doi.org/10.1136/oem.57.1.19
Devi C, Konduru KK (2018) Effect of cadmium exposure on cholinergic system and energy metabolism of rat brain: reversal effect of α-tocopherol. Int J Pharm Sci Res 50(1):373–381. https://doi.org/10.13040/IJPSR.0975-8232.9
Dési I, Nagymajtényi L, Schulz H (1998) Behavioural and neurotoxicological changes caused by cadmium treatment of rats during development. J ApplToxicol 18:63–70. https://doi.org/10.1002/(sici)1099-1263(199801/02)18:1<63::aid-jat475>3.0.co;2-z
Bar-Sela S, Reingold S, Richter ED (2001) Amyotrophic lateral sclerosis in a battery-factory worker exposed to cadmium. Int J Occup Environ Health 7(2):109–112. https://doi.org/10.1179/107735201800339470
Bao QJ, Zhao K, Guo Y, Wu XT, Yang JC, Yang MF (2022) Environmental toxic metal contaminants and risk of stroke: a systematic review and meta-analysis. Environ Sci Poll Res Int 29:32545–32565. https://doi.org/10.1007/s11356-022-18866-z
Vellingiri B, Suriyanarayanan A, Selvaraj P, Abraham KS, Pasha MY, Winster H, Gopalakrishnan AV, Reddy GS, Ayyadurai JK, Kumar N, Giridharan N, Rao BPS, Nachimuthu K, Narayanasamy SK, Mahalaxmi A, Venkatesan I D (2022) Role of heavy metals (copper (Cu), arsenic (as), cadmium (cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 301:134625. https://doi.org/10.1016/j.chemosphere.2022.134625
Amadi CN, Offor SJ, Frazzoli C, Orisakwe OE (2019) Natural antidotes and management of metal toxicity. Environ SciPollRes Int 26:18032–18052. https://doi.org/10.1007/s11356-019-05104-2
Pinheiro JEG, Martinez CS, Moraes PZ, Stasiaki JE, Trost ME, Vasallo DV, Barbosa FJ, Santos FW, Miguel M, Wiggers GA (2019) Egg white hydrolysate prevents reproductive impairments induced by cadmium in rats. J Func Foods 67:103823, 2020. https://doi.org/10.1016/j.jff.2020.103823
Moraes PZ, Pinheiro JEG, Martinez CS, Moro CR, da Silva GC, Rodriguez MD, Simoes MR, Barbosa FJ, Peçanha FM, Vasallo DV, Miguel M, Wiggers GA (2022) Multi-functional Egg White Hydrolysate prevent Hypertension and Vascular Dysfunction Induced by Cadmium in rats. J Funct Foods 94:105131. https://doi.org/10.1016/j.jff.2022.105131
Rizzetti DA, Altermann CD, Martinez CS, Peçanha FM, Vassallo DV, Uranga-Ocio JA, Castro MM, Wiggers GA, Mello-Carpes PB (2016) Ameliorative effects of egg white hydrolysate on recognition memory impairments associated with chronic exposure to low mercury concentration. Neurochem Int 101:30–37. https://doi.org/10.1016/j.neuint.2016.10.002
Martinez CS, Piagette JT, Escobar AG, Martín A, Palacios R, Peçanha FM, Vassallo DV, Exley C, Alonso MJ, Salaices M, Miguel M, Wiggers GA (2019) Egg White Hydrolysate: a new putative agent to prevent vascular dysfunction in rats following long-term exposure to aluminum. Food Chem Toxicol 133:110799. https://doi.org/10.1016/j.fct.2019.110799
Garcia-Redondo AB, Roque FR, Miguel M, López-Fandiño R, Salaices M (2010) Vascular effects of egg white-derived peptides in resistance arteries from rats. Structure-activity relationships. J Sci Food Agric 90:1988–1993. https://doi.org/10.1002/jsfa.4037
Miguel M, Recio I, Gómez-Ruiz JA, Ramos M, López-Fandiño R (2004) Angiotensin I-converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis. J Food Protect 67:1914–1920. https://doi.org/10.4315/0362-028x-67.9.1914
Miguel M, López-Fandiño R, Ramos M, Aleixandre A (2006) Long-term intake of egg white hydrolysate attenuates the development of hypertension in spontaneously hypertensive rats. Life Sci 78:2960–2966. https://doi.org/10.1016/j.lfs.2005.11.025
Garcés-Rimón M, González C, Uranga JA, López-Miranda V, López-Fandiño R, Miguel M (2016) Pepsin Egg White Hydrolysate ameliorates obesity-related oxidative stress, inflammation and steatosis in Zucker fatty rats. PLoS ONE 11:e0151193. https://doi.org/10.1371/journal.pone.0151193
Dávalos A, Miguel M, Bartolomé B, López-Fandiño R (2004) Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J Food Protect 67:1939–1944. https://doi.org/10.4315/0362-028x-67.9.1939
Manso MA, Miguel M, Even J, Hernández R, Aleixandre A, López-Fandiño R (2008) Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem 109:361–367. https://doi.org/10.1016/j.foodchem.2007.12.049
Martinez CS, Alterman C, Vera G, Márquez A, Uranga JA, Peçanha FM, Vassallo DV, Exley C, Mello-Carpes PB, Miguel M, Wiggers GA (2019) Egg White Hydrolysate as a functional food ingredient to prevent cognitive dysfunction in rats following long-term exposure to aluminum. Sci Rep 9:1868. https://doi.org/10.1038/s41598-018-38226-7
Balaraman R, Gulati OD, Bhatt JD, Rathod SP, Hemavathi KG (1989) Cadmium-induced hypertension in rats. Pharmacol 38_226–234. https://doi.org/10.1159/000138541
Miguel M, López-Fandiño R, Ramos M, Aleixandre A (2005) Short-term effect of egg-white hydrolysate products on the arterial blood pressure of hypertensive rats. Br Nutr Nutr 94:731–737. https://doi.org/10.1079/bjn20051570
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17:472–476. https://doi.org/10.1161/01.str.17.3.472
Sosa PM, Schimidt HL, Altermann C, Vieira AS, Cibin FW, Carpes FP, Mello-Carpes PB (2015) Physical exercise prevents motor disorders and striatal oxidative imbalance after cerebral ischemia-reperfusion. Braz J Med Biol Res 48:798–804. https://doi.org/10.1590/1414-431X20154429
Bonini JS, Bevilaqua LR, Zinn CG, Kerr DS, Medina JH, Izquierdo I, Cammarota M (2006) Angiotensin II disrupts inhibitory avoidance memory retrieval. Horm Behav 50:308–313. https://doi.org/10.1016/j.yhbeh.2006.03.016
Hunskaar S, Berge OG, Hole K (1986) A modified hot-plate test sensitive to mild analgesics. Behav Brain Res 21:101–108. https://doi.org/10.1016/0166-4328(86)90088-4
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167. https://doi.org/10.1016/0165-0270(85)90031-7
Loetchutinat C, Kothan S, Dechsupa S (2005) Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant. Radiat Phys Chem 72:323–331. https://doi.org/10.1016/j.radphyschem.2004.06.011
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:51–358. https://doi.org/10.1016/0003-2697(79)90738-3
Martinez CS, Escobar AG, Uranga-Ocio JA, Peçanha FM, Vassallo DV, Exley C, Miguel M, Wiggers GA (2017) Aluminum exposure for 60days at human dietary levels impairs spermatogenesis and sperm quality in rats. Reprod Toxicol 73:128–141. https://doi.org/10.1016/j.reprotox.2017.08.008
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. AnalBiochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175. https://doi.org/10.1016/S0021-9258(19)45228-9
Kruger NJ (1994) The Bradford method for protein quantitation. Methods Mol Biol 32:9–15. https://doi.org/10.1385/0-89603-268-X:9
Méndez-Armenta M, Ríos C (2007) Cadmium neurotoxicity. EnvironToxicolPharmacol 23:350–358. https://doi.org/10.1016/j.etap.2006.11.009
Haider S, Anis L, Batool Z, Sajid I, Naqvi F, Khaliq S, Ahmed S (2015) Short term cadmium administration dose dependently elicits immediate biochemical, neurochemical and neurobehavioral dysfunction in male rats. Metab Brain Dis 30–:83–92. https://doi.org/10.1007/s11011-014-9578-4
Gagnaire B, Adam-Guillermin C, Bouron A, Lestaevel P (2011) The effects of radionuclides on animal behavior. Rev Environ Contam Toxicol 210:35–58. https://doi.org/10.1007/978-1-4419-7615-4_2
Cai Y, Xu W, Wang M, Chen W, Li X, Li Y, Cai Y (2019) Mechanisms and uncertainties of Zn supply on regulating rice cd uptake. Environ Poll 253:959–965. https://doi.org/10.1016/j.envpol.2019.07.077
del Pino J, Zeballos G, Anadón MJ, Díaz MJ, García JM, Freijo MT (2016) Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels. Arch Toxicol 90:1081–1092. https://doi.org/10.1007/s00204-015-1540-7
Branca J, Maresca M, Morucci G, Mello T, Becatti M, Pazzagli L, Colzi I, Gonnelli C, Carrino D, Paternostro F, Nicoletti C, Ghelardini C, Gulisano M, Di Cesare Mannelli L, Pacini A (2019) Effects of Cadmium on ZO-1 tight Junction Integrity of the blood brain barrier. Int J Mol Sci 20:6010. https://doi.org/10.3390/ijms20236010
Pinheiro Júnior JEG, Moraes PZ, Rodriguez MD, Simões MR, Cibin F, Pinton S, Barbosa Junior F, Peçanha FM, Vassallo DV, Miguel M, Wiggers GA (2020) Cadmium exposure activates NADPH oxidase, renin-angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage. ToxicolLett 333:80–89. https://doi.org/10.1016/j.toxlet.2020.07.027
Branca J, Fiorillo C, Carrino D, Paternostro F, Taddei N, Gulisano M, Pacini A, Becatti M (2020) Cadmium-Induced oxidative stress: focus on the Central Nervous System. Antioxidants 9:492. https://doi.org/10.3390/antiox9060492
Wang Y, Fang J, Leonard SS, Rao KM (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Rad Biolo Med 36:1434–1443. https://doi.org/10.1016/j.freeradbiomed.2004.03.010
Agnihotri SK, Agrawal U, Ghosh I (2015) Brain most susceptible to cadmium induced oxidative stress in mice. J Trace Elem Med Biol 30:184–193. https://doi.org/10.1016/j.jtemb.2014.12.008
Kini RD, Arunkumar N, Sneha SB, Anupama N, Bhagyalakshmi K, Gokul M (2019) Potential protective role of beta carotene on cadmium induced brain and kidney damage. Indian J Public Health Res Dev 10:532–535. https://doi.org/10.5958/0976-5506.2019.02484.7
Chen S, Ren Q, Zhang J, Ye Y, Zhang Z, Xu Y, Guo M, Ji H, Xu C, Gu C, Gao W, Huang S, Chen L (2014) N-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brain. Neuropathol aAppl Neurobiol 40:759–777. https://doi.org/10.1111/nan.12103
Papp A, Oszlánczi G, Horváth E, Paulik E, Kozma G, Sápi A, Kónya Z, Szabó A (2012) Consequences of subacute intratracheal exposure of rats to cadmium oxide nanoparticles: electrophysiological and toxicological effects. Toxicol Ind Health 28:933–941. https://doi.org/10.1177/0748233711430973
Abdalla FH, Schmatz R, Cardoso AM, Carvalho FB, Baldissarelli J, de Oliveira JS, Rosa MM, Gonçalves Nunes MA, Rubin MA, da Cruz IB, Barbisan F, Dressler VL, Pereira LB, Schetinger MR, Morsch VM, Gonçalves JF, Mazzanti CM (2014) Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and na(+), K(+)-ATPase activities. Physiol Behav 135:152–167. https://doi.org/10.1016/j.physbeh.2014.06.008
Lamtai M, Chaibat J, Ouakki S, Berkkiks I, Rifi E (2018) Effect of Chronic Administration of Cadmium on Anxiety-Like, Depression-Like and Memory deficits in male and female rats: possible involvement of oxidative stress mechanism. J Behav Brain Sci 8:240–268. https://doi.org/10.4236/jbbs.2018.85016
Wang J, Chen X, Bai W, Wang Z, Xiao W, Zhu J (2021) Study on mechanism of Ginkgo biloba L. leaves for the treatment of neurodegenerative diseases based on Network Pharmacology. Neurochem Res 46:1881–1894. https://doi.org/10.1007/s11064-021-03315-z
Gibson EL, Vargas K, Hogan E, Holmes A, Rogers PJ, Wittwer J, Kloek J, Goralczyk R, Mohajeri MH (2014) Effects of acute treatment with a tryptophan-rich protein hydrolysate on plasma amino acids, mood and emotional functioning in older women. Psychopharmacol 231:4595–4610. https://doi.org/10.1007/s00213-014-3609-z
Zheng J, Bu T, Liu L, He G, Li S, Wu J (2020) Naturally occurring low molecular peptides identified in egg white show antioxidant activity. Food Res iInt 138:109766. https://doi.org/10.1016/j.foodres.2020.109766
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development - CNPq [Edital Universal/CNPq No 44181/2014-9]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES); Fundação de Amparo à Pesquisa do Rio Grande do Sul - FAPERGS/ Brazil [PQG:19/2551-0001810-0]; Programa Nacional de Cooperação Acadêmica; Pró-reitoria de Pesquisa - Universidade Federal do Pampa [N. 20180615102630]; FAPES/ CNPq/PRONEX [N. 80598773] and Spanish Goverment by the Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER) [AGL2017-89213]; I-COOP + 2020 (COOPA 20453). JEGPJ, PMS and BSN were supported by CAPES/Brazil. DVV, PBMC and GAW are research fellows from CNPq (308281/2021-7, 312239/2021-1 and 311834/2020-5).
Funding
This work was supported by the National Council for Scientific and Technological Development - CNPq [Edital Universal/CNPq No 44181/2014-9]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES); Fundação de Amparo à Pesquisa do Rio Grande do Sul - FAPERGS/ Brazil [PQG:19/2551-0001810-0]; Programa Nacional de Cooperação Acadêmica; Pró-reitoria de Pesquisa - Universidade Federal do Pampa [N. 20180615102630]; FAPES/ CNPq/PRONEX [N. 80598773] and Spanish Goverment by the Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER) [AGL2017-89213]; I-COOP + 2020 (COOPA 20453). JEGPJ, PMS and BSN were supported by CAPES/Brazil. DVV, PBMC and GAW are research fellows from CNPq (308281/2021-7, 312239/2021-1 and 311834/2020-5).
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. José Eudes Gomes Pinheiro Junior, Priscila Marques Sosa, and Ben-Hur Souto das Neves performed material preparation, data collection, and analysis. The first draft of the manuscript was written by José Eudes Gomes Pinheiro Junior, Ben-Hur Souto das Neves, Marta Miguel-Castro, Pâmela Billig Mello-Carpes, and Giulia Alessandra Wiggers, and all authors commented on previous versions of the manuscript. Finally, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
José Eudes Gomes Pinheiro Júnior and Priscila Marques Sosa contributed equally
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinheiro Júnior, J.E.G., Sosa, P.M., das Neves, BH.S. et al. Egg White Hydrolysate Mitigates Cadmium-induced Neurological Disorders and Oxidative Damage. Neurochem Res 49, 1603–1615 (2024). https://doi.org/10.1007/s11064-024-04110-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-024-04110-2