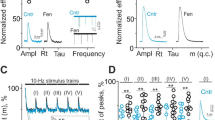
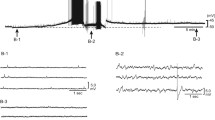
Abstract
α2-Adrenoreceptors (ARs) are main Gi-protein coupled autoreceptors in sympathetic nerve terminals and targets for dexmedetomidine (DEX), a widely used sedative. We hypothesize that α2-ARs are also potent regulators of neuromuscular transmission via G protein-gated inwardly rectifying potassium (GIRK) channels. Using extracellular microelectrode recording of postsynaptic potentials, we found DEX-induced inhibition of spontaneous and evoked neurotransmitter release as well as desynchronization of evoked exocytotic events in the mouse diaphragm neuromuscular junction. These effects were suppressed by SKF-86,466, a selective α2-AR antagonist. An activator of GIRK channels ML297 had the same effects on neurotransmitter release as DEX. By contrast, inhibition of GIRK channels with tertiapin-Q prevented the action of DEX on evoked neurotransmitter release, but not on spontaneous exocytosis. The synaptic vesicle exocytosis is strongly dependent on Ca2+ influx through voltage-gated Ca2+ channels (VGCCs), which can be negatively regulated via α2-AR – GIRK channel axis. Indeed, inhibition of P/Q-, L-, N- or R-type VGCCs prevented the inhibitory action of DEX on evoked neurotransmitter release; antagonists of P/Q- and N-type channels also suppressed the DEX-mediated desynchronization of evoked exocytotic events. Furthermore, inhibition of P/Q-, L- or N-type VGCCs precluded the frequency decrease of spontaneous exocytosis upon DEX application. Thus, α2-ARs acting via GIRK channels and VGCCs (mainly, P/Q- and N-types) exert inhibitory effect on the neuromuscular communication by attenuating and desynchronizing evoked exocytosis. In addition, α2-ARs can suppress spontaneous exocytosis through GIRK channel-independent, but VGCC-dependent pathway.




Similar content being viewed by others
Data Availability
All mentioned data are represented in the main manuscript figures and supplementary figures. Other additional data will be made available on reasonable request.
Abbreviations
- AR:
-
Adrenoceptor
- DEX:
-
Dexmedetomidine
- EPP:
-
End-plate potential
- GIRK:
-
Channel, G Protein-gated inwardly rectifying potassium channel
- MEPP:
-
Miniature end-plate potential
- NMJ:
-
Neuromuscular junction
- nAChR:
-
Nicotinic acetylcholine receptor
- TPQ:
-
Tertiapin-Q
- VGCC:
-
Voltage-gated Ca2+ channel
References
Wang ZM, Messi ML, Rodrigues ACZ, Delbono O (2022) Skeletal muscle sympathetic denervation disrupts the neuromuscular junction postterminal organization: a single-cell quantitative approach. Mol Cell Neurosci 120:103730
Dmitrieva SA, Vologin SG, Tsentsevitsky AN, Arkhipov AY, Khuzakhmetova VF, Sibgatullina GV, Bukharaeva EA (2023) Sympathetic innervation and endogenous catecholamines in neuromuscular preparations of muscles with different functional profiles. Biochem (Mosc) 88:364–373
Delbono O, Rodrigues ACZ, Bonilla HJ, Messi ML (2021) The emerging role of the sympathetic nervous system in skeletal muscle motor innervation and sarcopenia. Ageing Res Rev 67:101305
Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, O’Connor E, Cox D, Reischl M, Marquardt T, Labeit D, Labeit S, Benoit E, Molgo J, Lochmuller H, Witzemann V, Kettelhut IC, Navegantes LC, Pozzan T, Rudolf R (2016) Sympathetic innervation controls homeostasis of neuromuscular junctions in health and Disease. Proc Natl Acad Sci U S A 113:746–750
Petrov AM, Zakirjanova GF, Kovyazina IV, Tsentsevitsky AN, Bukharaeva EA (2022) Adrenergic receptors control frequency-dependent switching of the exocytosis mode between full-collapse and kiss-and-run in murine motor nerve terminal. Life Sci 296:120433
Straka T, Vita V, Prokshi K, Horner SJ, Khan MM, Pirazzini M, Williams MPI, Hafner M, Zaglia T, Rudolf R (2018) Postnatal Development and Distribution of Sympathetic Innervation in mouse skeletal muscle. Int J Mol Sci 19
Bukharaeva E, Khuzakhmetova V, Dmitrieva S, Tsentsevitsky A (2021) Adrenoceptors modulate cholinergic synaptic transmission at the Neuromuscular Junction. Int J Mol Sci 22
Wang ZM, Messi ML, Grinevich V, Budygin E, Delbono O (2020) Postganglionic sympathetic neurons, but not locus coeruleus optostimulation, activates neuromuscular transmission in the adult mouse in vivo. Mol Cell Neurosci 109:103563
Tsentsevitsky A, Nurullin L, Tyapkina O, Bukharaeva E (2020) Sympathomimetics regulate quantal acetylcholine release at neuromuscular junctions through various types of adrenoreceptors. Mol Cell Neurosci 108:103550
Wang ZM, Rodrigues ACZ, Messi ML, Delbono O (2020) Aging blunts sympathetic Neuron Regulation of motoneurons synaptic vesicle release mediated by beta1- and alpha2B-Adrenergic receptors in geriatric mice. J Gerontol A Biol Sci Med Sci 75:1473–1480
Clausen L, Cossins J, Beeson D (2018) Beta-2 adrenergic receptor agonists enhance AChR Clustering in C2C12 myotubes: implications for Therapy of Myasthenic disorders. J Neuromuscul Dis 5:231–240
Li S, Sun B, Nilsson MI, Bird A, Tarnopolsky MA, Thurberg BL, Bali D, Koeberl DD (2013) Adjunctive beta2-agonists reverse neuromuscular involvement in murine pompe Disease. FASEB J 27:34–44
Ghazanfari N, Morsch M, Tse N, Reddel SW, Phillips WD (2014) Effects of the ss2-adrenoceptor agonist, albuterol, in a mouse model of anti-MuSK myasthenia gravis. PLoS ONE 9:e87840
Bartus RT, Betourne A, Basile A, Peterson BL, Glass J, Boulis NM (2016) beta2-Adrenoceptor agonists as novel, safe and potentially effective therapies for Amyotrophic Lateral Sclerosis (ALS). Neurobiol Dis 85:11–24
McMacken GM, Spendiff S, Whittaker RG, O’Connor E, Howarth RM, Boczonadi V, Horvath R, Slater CR, Lochmuller H (2019) Salbutamol modifies the neuromuscular junction in a mouse model of ColQ myasthenic syndrome. Hum Mol Genet 28:2339–2351
Rodriguez Cruz PM, Cossins J, Cheung J, Maxwell S, Jayawant S, Herbst R, Waithe D, Kornev AP, Palace J, Beeson D (2020) Congenital myasthenic syndrome due to mutations in MUSK suggests that the level of MuSK phosphorylation is crucial for governing synaptic structure. Hum Mutat 41:619–631
Breuer T, Bleilevens C, Rossaint R, Marx G, Gehrenkemper J, Dierksen H, Delpierre A, Weis J, Gayan-Ramirez G, Bruells CS (2018) Dexmedetomidine impairs diaphragm function and increases oxidative stress but does not aggravate diaphragmatic atrophy in mechanically ventilated rats. Anesthesiology 128:784–795
Cheng W, Wu Z, Zhang J, Ren W (2023) Effect of dexmedetomidine on tourniquet-induced skeletal muscle injury. Rev Assoc Med Bras (1992) 69:228–232
Liu M, Liu Y, Li X, Pei M, Han M, Qi F (2022) Dexmedetomidine inhibits abnormal muscle hypertrophy of myofascial trigger points via TNF-alpha/ NF-kappaB signaling pathway in rats. Front Pharmacol 13:1031804
Haouzi P, Tubbs N (2022) Effects of fentanyl overdose-induced muscle rigidity and dexmedetomidine on respiratory mechanics and pulmonary gas exchange in sedated rats. J Appl Physiol (1985) 132:1407–1422
Kundra TS, Thimmarayappa A, Dhananjaya M, Manjunatha N (2018) Dexmedetomidine for prevention of skeletal muscle ischaemia-reperfusion injury in patients with chronic limb ischaemia undergoing aortobifemoral bypass Surgery: a prospective double-blind randomized controlled study. Ann Card Anaesth 21:22–25
Cheng M, Gao T, Xi F, Cao C, Chen Y, Zhao C, Li Q, Yu W (2017) Dexmedetomidine ameliorates muscle wasting and attenuates the alteration of hypothalamic neuropeptides and inflammation in endotoxemic rats. PLoS ONE 12:e0174894
Li SP, Zhou XL, Zhao Y (2020) Sedation with midazolam worsens the diaphragm function than dexmedetomidine and propofol during mechanical ventilation in rats. Biomed Pharmacother 121:109405
Weinger MB, Partridge BL, Henry AF (1995) Dexmedetomidine does not modify the neuromuscular blocking action of vecuronium in the anaesthetized rat. Br J Anaesth 74:455–457
Sugita S, Fleming LL, Wood C, Vaughan SK, Gomes MP, Camargo W, Naves LA, Prado VF, Prado MA, Guatimosim C, Valdez G (2016) VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions. Skelet Muscle 6:31
Zakyrjanova GF, Giniatullin AR, Mukhutdinova KA, Kuznetsova EA, Petrov AM (2021) Early differences in membrane properties at the neuromuscular junctions of ALS model mice: effects of 25-hydroxycholesterol. Life Sci 273:119300
Nervo A, Calas AG, Nachon F, Krejci E (2019) Respiratory Failure triggered by cholinesterase inhibitors may involve activation of a reflex sensory pathway by acetylcholine spillover. Toxicology 424:152232
Cisterna BA, Vargas AA, Puebla C, Fernandez P, Escamilla R, Lagos CF, Matus MF, Vilos C, Cea LA, Barnafi E, Gaete H, Escobar DF, Cardozo CP, Saez JC (2020) Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat Commun 11:1073
Kavalali ET (2020) Neuronal ca(2+) signalling at rest and during spontaneous neurotransmission. J Physiol 598:1649–1654
Homan AE, Meriney SD (2018) Active zone structure-function relationships at the neuromuscular junction. Synapse 72:e22057
Tsentsevitsky AN, Gafurova CR, Mukhutdinova KA, Giniatullin AR, Fedorov NS, Malomouzh AI, Petrov AM (2023) Sphingomyelinase modulates synaptic vesicle mobilization at the mice neuromuscular junctions. Life Sci 318:121507
Khuzakhmetova V, Bukharaeva E (2021) Adrenaline facilitates synaptic transmission by Synchronizing Release of Acetylcholine Quanta from Motor nerve endings. Cell Mol Neurobiol 41:395–401
Del Castillo J, Katz B (1954) The effect of magnesium on the activity of motor nerve endings. J Physiol 124:553–559
Bukcharaeva EA, Kim KC, Moravec J, Nikolsky EE, Vyskocil F (1999) Noradrenaline synchronizes evoked quantal release at frog neuromuscular junctions. J Physiol 517(Pt 3):879–888
Tsentsevitsky AN, Petrov AM (2022) L-type ca(2+) channels at Low External Calcium differentially regulate neurotransmitter release in proximal-distal compartments of the Frog Neuromuscular Junction. Cell Mol Neurobiol 42:2833–2847
Mikami M, Zhang Y, Kim B, Worgall TS, Groeben H, Emala CW (2017) Dexmedetomidine’s inhibitory effects on acetylcholine release from cholinergic nerves in guinea pig trachea: a mechanism that accounts for its clinical benefit during airway irritation. BMC Anesthesiol 17:52
Protti DA, Uchitel OD (1993) Transmitter release and presynaptic Ca2 + currents blocked by the spider toxin omega-Aga-IVA. NeuroReport 5:333–336
Katz E, Ferro PA, Weisz G, Uchitel OD (1996) Calcium channels involved in synaptic transmission at the mature and regenerating mouse neuromuscular junction. J Physiol 497(Pt 3):687–697
Protti DA, Szczupak L, Scornik FS, Uchitel OD (1991) Effect of omega-conotoxin GVIA on neurotransmitter release at the mouse neuromuscular junction. Brain Res 557:336–339
Almog M, Korngreen A (2009) Characterization of voltage-gated ca(2+) conductances in layer 5 neocortical pyramidal neurons from rats. PLoS ONE 4:e4841
Kaufmann K, Romaine I, Days E, Pascual C, Malik A, Yang L, Zou B, Du Y, Sliwoski G, Morrison RD, Denton J, Niswender CM, Daniels JS, Sulikowski GA, Xie XS, Lindsley CW, Weaver CD (2013) ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci 4:1278–1286
Tsentsevitsky AN, Khaziev EF, Kovyazina IV, Petrov AM (2022) GIRK channel as a versatile regulator of neurotransmitter release via L-type ca(2+) channel-dependent mechanism in the neuromuscular junction. Neuropharmacology 209:109021
Bukharaeva EA, Skorinkin AI, Samigullin DV, Petrov AM (2022) Presynaptic acetylcholine receptors modulate the Time Course of Action Potential-Evoked Acetylcholine Quanta Secretion at Neuromuscular junctions. Biomedicines 10
Bunemann M, Bucheler MM, Philipp M, Lohse MJ, Hein L (2001) Activation and deactivation kinetics of alpha 2A- and alpha 2 C-adrenergic receptor-activated G protein-activated inwardly rectifying K + channel currents. J Biol Chem 276:47512–47517
Luscher C, Slesinger PA (2010) Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and Disease. Nat Rev Neurosci 11:301–315
Luo H, Marron Fernandez de Velasco E, Wickman K (2022) Neuronal G protein-gated K(+) channels. Am J Physiol Cell Physiol 323:C439–C460
Jin W, Lu Z (1998) A novel high-affinity inhibitor for inward-rectifier K + channels. Biochemistry 37:13291–13299
Zurawski Z, Thompson Gray AD, Brady LJ, Page B, Church E, Harris NA, Dohn MR, Yim YY, Hyde K, Mortlock DP, Jones CK, Winder DG, Alford S, Hamm HE (2019) Disabling the Gbetagamma-SNARE interaction disrupts GPCR-mediated presynaptic inhibition, leading to physiological and behavioral phenotypes. Sci Signal 12
Dolphin AC (1998) Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J Physiol 506(Pt 1):3–11
Urbano FJ, Rosato-Siri MD, Uchitel OD (2002) Calcium channels involved in neurotransmitter release at adult, neonatal and P/Q-type deficient neuromuscular junctions (review). Mol Membr Biol 19:293–300
Yang L, Tang J, Dong J, Zheng J (2015) Alpha2-adrenoceptor-independent inhibition of acetylcholine receptor channel and sodium channel by dexmedetomidine in rat superior cervical ganglion neurons. Neuroscience 289:9–18
Cui LN, Sun N, Li BX, Wang LF, Zhang XY, Qiu DL, Chu CP (2020) Noradrenaline inhibits complex spikes activity via the presynaptic PKA signaling pathway in mouse cerebellar slices. Neurosci Lett 729:135008
Dong CJ, Guo Y, Ye Y, Hare WA (2014) Presynaptic inhibition by alpha2 receptor/adenylate cyclase/PDE4 complex at retinal rod bipolar synapse. J Neurosci 34:9432–9440
DeBock F, Kurz J, Azad SC, Parsons CG, Hapfelmeier G, Zieglgansberger W, Rammes G (2003) Alpha2-adrenoreceptor activation inhibits LTP and LTD in the basolateral amygdala: involvement of Gi/o-protein-mediated modulation of Ca2+-channels and inwardly rectifying K+-channels in LTD. Eur J Neurosci 17:1411–1424
Conductier G, Nahon JL, Guyon A (2011) Dopamine depresses melanin concentrating hormone neuronal activity through multiple effects on alpha2-noradrenergic, D1 and D2-like dopaminergic receptors. Neuroscience 178:89–100
Alberto CO, Trask RB, Hirasawa M (2011) Dopamine acts as a partial agonist for alpha2A adrenoceptor in melanin-concentrating hormone neurons. J Neurosci 31:10671–10676
Tsentsevitsky AN, Gafurova CR, Petrov AM (2022) K(ATP) channels as ROS-dependent modulator of neurotransmitter release at the neuromuscular junctions. Life Sci 310:121120
Crawford DC, Kavalali ET (2015) Molecular underpinnings of synaptic vesicle pool heterogeneity. Traffic 16:338–364
Melom JE, Akbergenova Y, Gavornik JP, Littleton JT (2013) Spontaneous and evoked release are independently regulated at individual active zones. J Neurosci 33:17253–17263
Robitaille R (1998) Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron 21:847–855
Ladera C, del Carmen Godino M, Jose Cabanero M, Torres M, Watanabe M, Lujan R, Sanchez-Prieto J (2008) Pre-synaptic GABA receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J Neurochem 107:1506–1517
Vazquez-Vazquez H, Gonzalez-Sandoval C, Vega AV, Arias-Montano JA, Barral J (2022) Histamine H(3) receptor activation modulates glutamate release in the Corticostriatal Synapse by acting at ca(V)2.1 (P/Q-Type) calcium channels and GIRK (K(IR)3) Potassium channels. Cell Mol Neurobiol 42:817–828
Guo J, Ikeda SR (2004) Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol 65:665–674
Ginebaugh SP, Badawi Y, Tarr TB, Meriney SD (2022) Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States. Biomolecules 12
Nishimune H, Badawi Y, Mori S, Shigemoto K (2016) Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice. Sci Rep 6:27935
Giovannini F, Sher E, Webster R, Boot J, Lang B (2002) Calcium channel subtypes contributing to acetylcholine release from normal, 4-aminopyridine-treated and myasthenic syndrome auto-antibodies-affected neuromuscular junctions. Br J Pharmacol 136:1135–1145
Kaja S, Van de Ven RC, Ferrari MD, Frants RR, Van den Maagdenberg AM, Plomp JJ (2006) Compensatory contribution of Cav2.3 channels to acetylcholine release at the neuromuscular junction of tottering mice. J Neurophysiol 95:2698–2704
Pardo NE, Hajela RK, Atchison WD (2006) Acetylcholine release at neuromuscular junctions of adult tottering mice is controlled by N-(cav2.2) and R-type (cav2.3) but not L-type (cav1.2) Ca2 + channels. J Pharmacol Exp Ther 319:1009–1020
Molina-Campos E, Xu Y, Atchison WD (2015) Age-dependent contribution of P/Q- and R-type Ca2 + channels to neuromuscular transmission in lethargic mice. J Pharmacol Exp Ther 352:395–404
Pagani R, Song M, McEnery M, Qin N, Tsien RW, Toro L, Stefani E, Uchitel OD (2004) Differential expression of alpha 1 and beta subunits of voltage dependent Ca2 + channel at the neuromuscular junction of normal and P/Q Ca2 + channel knockout mouse. Neuroscience 123:75–85
Todorov B, van de Ven RC, Kaja S, Broos LA, Verbeek SJ, Plomp JJ, Ferrari MD, Frants RR, van den Maagdenberg AM (2006) Conditional inactivation of the Cacna1a gene in transgenic mice. Genesis 44:589–594
Perissinotti PP, Giugovaz Tropper B, Uchitel OD (2008) L-type calcium channels are involved in fast endocytosis at the mouse neuromuscular junction. Eur J Neurosci 27:1333–1344
Depetris RS, Nudler SI, Uchitel OD, Urbano FJ (2008) Altered synaptic synchrony in motor nerve terminals lacking P/Q-calcium channels. Synapse 62:466–471
Urbano FJ, Piedras-Renteria ES, Jun K, Shin HS, Uchitel OD, Tsien RW (2003) Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2 + channels. Proc Natl Acad Sci U S A 100:3491–3496
Gilmanov IR, Samigullin DV, Vyskocil F, Nikolsky EE, Bukharaeva EA (2008) Modeling of quantal neurotransmitter release kinetics in the presence of fixed and mobile calcium buffers. J Comput Neurosci 25:296–307
Wang X, Pinter MJ, Rich MM (2010) Ca2 + dependence of the binomial parameters p and n at the mouse neuromuscular junction. J Neurophysiol 103:659–666
Ermolyuk YS, Alder FG, Surges R, Pavlov IY, Timofeeva Y, Kullmann DM, Volynski KE (2013) Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2 + channels. Nat Neurosci 16:1754–1763
Kailey B, van de Bunt M, Cheley S, Johnson PR, MacDonald PE, Gloyn AL, Rorsman P, Braun M (2012) SSTR2 is the functionally dominant somatostatin receptor in human pancreatic beta- and alpha-cells. Am J Physiol Endocrinol Metab 303:E1107–1116
Kuzhikandathil EV, Oxford GS (1999) Activation of human D3 dopamine receptor inhibits P/Q-type calcium channels and secretory activity in AtT-20 cells. J Neurosci 19:1698–1707
Zamponi GW, Currie KP (2013) Regulation of ca(V)2 calcium channels by G protein coupled receptors. Biochim Biophys Acta 1828:1629–1643
Acknowledgements
ANT, VFK were supported by assignment for Kazan Institute of Biochemistry and Biophysics, FRC Kazan Scientific Center of Russian Academy of Sciences.
Funding
This work was supported by the by Russian Science Foundation, grant number [23-15-00124], https://www.rscf.ru/project/23-15-00124/.
Author information
Authors and Affiliations
Contributions
A.T. performed electrophysiological experiments, visualization, and data analysis. V.K. performed electrophysiological experiments. EB was responsible for conceptualization, methodology, project administration, supervision, writing, review & editing; AMP wrote the main text and was responsible for conceptualization, review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. All authors consent to publish the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsentsevitsky, A.N., Khuzakhmetova, V.F., Bukharaeva, E.A. et al. The Mechanism of α2 adrenoreceptor-dependent Modulation of Neurotransmitter Release at the Neuromuscular Junctions. Neurochem Res 49, 453–465 (2024). https://doi.org/10.1007/s11064-023-04052-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04052-1