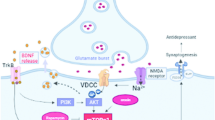
Abstract
The current first-line antidepressants have the drawback of slow onset, which greatly affects the treatment of depression. Crocetin, one of the main active ingredients in saffron (Crocus sativus L.), has been demonstrated to have antidepressant activities, but whether it has a rapid antidepressant effect remains unclear. This study aimed to investigate the onset, duration, and mechanisms of the rapid antidepressant activity of crocetin (20, 40 and 80 mg/kg, intraperitoneal injection) in male mice subjected to chronic restraint stress (CRS). The results of behavioral tests showed that crocetin exerted rapid antidepressant-like effect in mice with depression-like phenotypes, including rapid normalization of depressive-like behaviors within 3 h, and the effects could be maintained for 2 days. Hematoxylin-eosin (HE) and Nissl staining showed that crocetin ameliorated hippocampal neuroinflammation and nerve injuries in mice with depression-like phenotypes. The levels of inflammatory factors, corticosterone and pro brain-derived neurotrophic factor in crocetin-administrated mice serum were significantly reduced compared with those in the CRS group, as well as the levels of inflammatory factors in hippocampus. What’s more, Western blot analyses showed that, compared to CRS-induced mice, the relative levels of mitogen-activated kinase phosphatase 1 and toll-like receptor 4 were significantly reduced after the administration of crocetin, and the relative expressions of extracellular signal-regulated kinase 1/2 (ERK1/2), cAMP-response element binding protein, phosphorylated phosphoinositide 3 kinase (p-PI3K)/PI3K, phosphorylated protein kinase B (p-AKT)/AKT, phosphorylated glycogen synthase kinase 3β (p-GSK3β)/GSK3β, phosphorylated mammalian target of rapamycin (p-mTOR)/mTOR were markedly upregulated. In conclusion, crocetin exerted rapid antidepressant effects via suppressing the expression of inflammatory cytokines and the apoptosis of neuronal cells through PI3K/AKT signaling pathways. The rapid antidepressant effect of crocetin (40 mg/kg) could be maintained for at least 2 days after single treatment.







Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Marwaha S, Palmer E, Suppes T et al (2023) Novel and emerging treatments for major depression. Lancet 401(10371):141–153
Anthes E (2014) Depression: a change of mind. Nature 515(7526):185–187
Wilkinson ST, Sanacora G (2019) A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today 24(2):606–615
Bauer M, Severus E, Kohler S et al (2015) World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry 16(2):76–95
Shelton RC, Osuntokun O, Heinloth AN et al (2010) Therapeutic options for treatment-resistant depression. CNS Drugs 24(2):131–161
Gould TD, Zarate CA Jr, Thompson SM (2019) Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol 59:213–236
Sales AJ, Fogaca MV, Sartim AG et al (2019) Cannabidiol induces rapid and sustained antidepressant-like effects through increased bdnf signaling and synaptogenesis in the prefrontal cortex. Mol Neurobiol 56(2):1070–1081
Yin S, Shao J, Wang X et al (2019) Methylene blue exerts rapid neuroprotective effects on lipopolysaccharide-induced behavioral deficits in mice. Behav Brain Res 356:288–294
Ren Z, Yan P, Zhu L et al (2018) Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacology 235(1):233–244
Tang J, Xue W, Xia B et al (2015) Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci Rep 5:13573–13586
Sperner-Unterweger B, Kohl C, Fuchs D (2014) Immune changes and neurotransmitters: possible interactions in depression? Prog Neuro-Psychopharmacol Biol Psychiatry 48:268–276
Jin Y, Sun LH, Yang W et al (2019) The role of bdnf in the neuroimmune axis regulation of mood disorders. Front Neurol 10:515
Lee S, Kim HB, Hwang ES et al (2018) Antidepressant-like effects of p-coumaric acid on LPS-induced depressive and inflammatory changes in rats. Exp Neurobiol 27(3):189–199
Panczyszyn-Trzewik P, Misztak P, Nowak G et al (2017) Alterations of Nrf2 nuclear factor are associated with inflammation and oxidative stress in chronic mild stress animal model of depression. Eur Neuropsychopharmacol 27:S841–S841
Sowa-Kucma M, Styczen K, Siwek M et al (2018) Lipid peroxidation and Immune biomarkers are associated with major depression and its phenotypes, including treatment-resistant depression and melancholia. Neurotox Res 33(2):448–460
Lindqvist D, Dhabhar FS, James SJ et al (2017) Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 76:197–205
Birur B, Amrock EM, Shelton RC et al (2017) Sex differences in the peripheral immune system in patients with depression. Front Psychiatry 8:108
Pazini FL, Cunha MP, Rosa JM et al (2016) Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via pi3k/akt/mtor pathway. Mol Neurobiol 53(10):6818–6834
Duman RS, Voleti B (2012) Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35(1):47–56
Jin Y, Sui HJ, Dong Y et al (2012) Atorvastatin enhances neurite outgrowth in cortical neurons in vitro via up-regulating the Akt/mTOR and Akt/GSK-3 beta signaling pathways. Acta Pharmacol Sin 33(7):861–872
Neis VB, Moretti M, Rosa PB et al (2020) The involvement of PI3K/Akt/mTOR/GSK3 beta signaling pathways in the antidepressant-like effect of AZD6765. Pharmacol Biochem Behav 198:173020
Li NX, Lee B, Liu RJ et al (2010) mTOR-Dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994):959–964
Zarate CA (2020) Ketamine: a new chapter in antidepressant development. Braz J Psychiat 42(6):581–582
Gai M, Bo Q, Qi L (2016) Epigenetic down-regulated DDX10 promotes cell proliferation through Akt/NF-kappaB pathway in Ovarian cancer. Biochem Biophys Res Commun 469(4):1000–1005
Zhang C, Zeng M, Zhou L et al (2018) Baicalin exerts neuroprotective effects via inhibiting activation of GSK3beta/NF-kappaB/NLRP3 signal pathway in a rat model of depression. Int Immunopharmacol 64:175–182
Beurel E, Song L, Jope RS (2011) Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 16(11):1068–1070
Yang C, Zhou ZQ, Gao ZQ et al (2013) Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase-3beta, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol Psychiatry 73(12):e35-36
Mousavi SZ, Bathaie SZ (2011) Historical uses of saffron: identifying potential new avenues for modern research. Avicenna J Phytomed 1(2):57–66
Barbara T, Péter H, Tamás L et al (2019) The efficacy of saffron in the treatment of mild to moderate depression: a meta-analysis. Planta Med 85:24–31
Yoshino F, Yoshida A, Umigai N et al (2011) Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J Clin Biochem Nutr 49(3):182–187
Hong Y-J, Yang K-S (2013) Anti-inflammatory activities of crocetin derivatives from processed Gardenia jasminoides. Arch Pharm Res 36:933–940
Farkhondeh T, Samarghandian S, Samini F et al (2018) Protective effects of crocetin on depression-like behavior induced by immobilization in rat. CNS Neurol Disord 17(5):361–369
Mizuma H, Tanaka M, Nozaki S et al (2009) Daily oral administration of crocetin attenuates physical fatigue in human subjects. Nutr Res 29(3):145–150
Xi L, Zhiyu Q, Peng D et al (2007) Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine 14(9):633–636
Salama RM, Abdel-Latif GA, Abbas SS et al (2020) Neuroprotective effect of crocin against rotenone-induced Parkinson’s disease in rats: interplay between PI3K/Akt/mTOR signaling pathway and enhanced expression of miRNA-7 and miRNA-221. Neuropharmacology 164:107900
Lin S, Li Q, Xu Z et al (2022) Detection of the role of intestinal flora and tryptophan metabolism involved in antidepressant-like actions of crocetin based on a multi-omics approach. Psychopharmacology 239(11):3657–3677
Lin S, Li Q, Jiang S et al (2021) Crocetin ameliorates chronic restraint stress-induced depression-like behaviors in mice by regulating MEK/ERK pathways and gut microbiota. J Ethnopharmacol 268:113608
Chryssanthi DG, Dedesz PG, Karamanosz NK et al (2011) Crocetin inhibits invasiveness of MDA-MB-231 Breast cancer cells via downregulation of matrix metalloproteinases. Planta Med 77(2):146–151
Hu QY, Jin J, Zhou HF et al (2018) Crocetin attenuates DHT-induced polycystic ovary syndrome in mice via revising kisspeptin neurons. Biomed Pharmacother 107:1363–1369
Ishizuka F, Shimazawa M, Umigai N et al (2013) Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol 703(1–3):1–10
Yang RH, Yang LN, Shen XC et al (2012) Suppression of NF-kappa B pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol 674(2–3):391–396
Dulawa SC, Hollick KA, Gundersen B et al (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29(7):1321–1330
Christiansen SH, Olesen MV, Wortwein G et al (2011) Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav Brain Res 216(2):585–591
Zhao J, Jung YH, Jin Y et al (2019) A comprehensive metabolomics investigation of hippocampus, serum, and feces affected by chronic fluoxetine treatment using the chronic unpredictable mild stress mouse model of depression. Sci Rep 9:7566
Chen YM, Zhou H, An YJ et al (2019) Combined effects of olfactory dysfunction and chronic stress on anxiety- and depressive-like behaviors in mice. Neurosci Lett 692:143–149
Khalid A, Kim BS, Seo BA et al (2016) Gamma oscillation in functional brain networks is involved in the spontaneous remission of depressive behavior induced by chronic restraint stress in mice. BMC Neurosci 17:4
Aboul-Fotouh S (2013) Chronic treatment with coenzyme Q10 reverses restraint stress-induced anhedonia and enhances brain mitochondrial respiratory chain and creatine kinase activities in rats. Behav Pharmacol 24(7):552–560
Chiba S, Numakawa T, Ninomiya M et al (2012) Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro-Psychopharmacol Biol Psychiatry 39(1):112–119
Voorhees JL, Tarr AJ, Wohleb ES et al (2013) Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS ONE 8(3):e58488
Wang DN, Wu JB, Zhu PL et al (2022) Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: the potential involvement of gut-brain axis. Food Res Int 157:111289
Dang RZ, Wang MY, Li XH et al (2022) Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflam 19(1):41
Jiang JJ, Fu YY, Tang AY et al (2023) Sex difference in prebiotics on gut and blood-brain barrier dysfunction underlying stress-induced anxiety and depression. CNS Neurosci Ther. https://doi.org/10.1111/cns.14091
Zhang L, Chen ZW, Yang SF et al (2019) Micro RNA-219 decreases hippocampal long-term potentiation inhibition and hippocampal neuronal cell apoptosis in type 2 diabetes mellitus mice by suppressing the NMDAR signaling pathway. CNS Neurosci Ther 25(1):69–77
Liu A-H, Chu M, Wang Y-P (2019) Up-regulation of Trem2 inhibits hippocampal neuronal apoptosis and alleviates oxidative stress in Epilepsy via the PI3K/Akt pathway in mice. Neurosci Bull 35(3):471–485
Howe CL, LaFrance-Corey RG, Sundsbak RS et al (2012) Inflammatory monocytes damage the hippocampus during acute picornavirus Infection of the brain. J Neuroinflam 9:50
Gouirand AM, Matuszewich L (2005) The effects of chronic unpredictable stress on male rats in the water maze. Physiol Behav 86(1–2):21–31
Xu B, Lang L-m, Li S-Z et al (2019) Cortisol excess-mediated mitochondrial damage Induced hippocampal neuronal apoptosis in mice following cold exposure. Cells 8(6):612
Kuang WH, Dong ZQ, Tian LT et al (2018) IGF-1 defends against chronic-stress induced depression in rat models of chronic unpredictable mild stress through the PI3K/Akt/FoxO3a pathway. Kaohsiung J Med Sci 34(7):370–376
Amin B, Nakhsaz A, Hosseinzadeh H (2015) Evaluation of the antidepressant-like effects of acute and sub-acute administration of crocin and crocetin in mice. Avicenna J Phytomed 5(5):458–468
Zhang K, Wang Z, Pan X et al (2020) Antidepressant-like effects of xiaochaihutang in perimenopausal mice. J Ethnopharmacol 248:112318
Chen P, Li XF, Yu Y et al (2023) Administration time and dietary patterns modified the effect of inulin on CUMS-induced anxiety and depression. Mol Nutr Food Res 67(8):2200566
Zou LL, Tian YL, Wang YF et al (2023) High-cholesterol diet promotes depression- and anxiety-like behaviors in mice by impact gut microbe and neuroinflammation. J Affect Disord 327:425–438
Ma M, Ren Q, Yang C et al (2016) Adjunctive treatment of brexpiprazole with fluoxetine shows a rapid antidepressant effect in social defeat stress model: role of BDNF-TrkB signaling. Sci Rep 6:39209
Wu RY, Zhu DD, Xia YC et al (2015) A role of Yueju in fast-onset antidepressant action on major depressive disorder and serum BDNF expression: a randomly double-blind, fluoxetine-adjunct, placebo-controlled, pilot clinical study. Neuropsychiatr Dis Treat 11:2013–2021
Li Y, Luo YM, Tang J et al (2021) The positive effects of running exercise on hippocampal astrocytes in a rat model of depression. Transl Psychiatry 11(1):83
Shen J, Yang L, Wei WS (2021) Role of Fto on CaMKII/CREB signaling pathway of hippocampus in depressive-like behaviors induced by chronic restraint stress mice. Behav Brain Res 406:113227
Schoenfeld TJ, McCausland HC, Morris HD et al (2017) Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatry 82(12):914–923
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23
Sasi M, Vignoli B, Canossa M et al (2017) Neurobiology of local and intercellular BDNF signaling. Pflug Arch Eur J Phy 469(5–6):593–610
Li J, Chen J, Ma N et al (2019) Effects of corticosterone on the expression of mature brain-derived neurotrophic factor (mBDNF) and proBDNF in the hippocampal dentate gyrus. Behav Brain Res 365:150–156
Kato TA, Yamauchi Y, Horikawa H et al (2013) Neurotransmitters, psychotropic drugs and microglia: clinical implications for psychiatry. Curr Med Chem 20(3):331–344
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65(9):732–741
Zhang R-Y, Guo L-T, Ji Z-Y et al (2018) Radix Scutellariae attenuates cums-induced depressive-like behavior by promoting neurogenesis via CAMP/PKA pathway. Neurochem Res 43(11):2111–2120
Huang Y-L, Zeng N-X, Chen J et al (2020) Dynamic changes of behaviors, dentate gyrus neurogenesis and hippocampal miR-124 expression in rats with depression induced by chronic unpredictable mild stress. Neural Regener Res 15(6):1150–1159
Forlenza MJ, Miller GE (2006) Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med 68(1):1–7
Zhu C, Blakely RD, Hewlett WA (2006) The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31(10):2121–2131
Li K, Yan L, Zhang Y et al (2020) Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function. J Ethnopharmacol 250:112487
Das M, Das S, Das DK (2007) Caveolin and MAP kinase interaction in angiotensin II preconditioning of the myocardium. J Cell Mol Med 11(4):788–797
Das S, Otani H, Maulik N et al (2006) Redox regulation of angiotensin II preconditioning of the myocardium requires MAP kinase signaling. J Mol Cell Cardiol 41(2):248–255
Barthas F, Humo M, Gilsbach R et al (2017) Cingulate overexpression of mitogen-activated protein kinase phosphatase-1 as a key factor for depression. Biol Psychiatry 82(5):370–379
Wang CH, Zhang XL, Li Y et al (2015) Role of hippocampus mitogen-activated protein kinase phosphatase-1 mRNA expression and DNA methylation in the depression of the rats with chronic unpredicted stress. Cell Mol Neurobiol 35(4):473–482
Hui LY, Wang YW, Zhou FL et al (2016) Association between MKP-1, BDNF, and gonadal hormones with depression on perimenopausal women. J Womens Health 25(1):71–77
Hetman M, Gozdz A (2004) Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem 271(11):2050–2055
Walton M, Dragunow M (2000) Is CREB a key to neuronal survival. Trends Neurosci 23(2):48–53
Qi XL, Lin WJ, Li JF et al (2008) Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis 31(2):278–285
Cunha MP, Budni J, Ludka FK et al (2016) Involvement of pi3k/akt signaling pathway and its downstream intracellular targets in the antidepressant-like effect of creatine. Mol Neurobiol 53(5):2954–2968
Urbanska M, Gozdz A, Macias M et al (2018) Gsk3beta controls mtor and prosurvival signaling in neurons. Mol Neurobiol 55(7):6050–6062
Song B, Lai B, Zheng Z et al (2010) Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival. J Biol Chem 285(52):41122–41134
Gomez-Sintes R, Hernandez F, Lucas JJ et al (2011) Gsk-3 mouse models to study neuronal apoptosis and neurodegeneration. Front Mol Neurosci 4:45
Ludka FK, Constantino LC, Dal-Cim T et al (2016) Involvement of PI3K/Akt/GSK-3beta and mTOR in the antidepressant-like effect of atorvastatin in mice. J Psychiatr Res 82:50–57
Dalmagro AP, Camargo A, Severo Rodrigues AL et al (2019) Involvement of PI3K/Akt/GSK-3beta signaling pathway in the antidepressant-like and neuroprotective effects of Morus nigra and its major phenolic, syringic acid. Chem Biol Interact 314:108843
Chandran A, Iyo AH, Jernigan CS et al (2013) Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuro-Psychopharmacol Biol Psychiatry 40:240–245
Dwyer JM, Lepack AE, Duman RS (2012) mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol 15(4):429–434
Duman RS, Li N, Liu RJ et al (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62(1):35–41
Abelaira HM, Reus GZ, Neotti MV et al (2014) The role of mTOR in depression and antidepressant responses. Life Sci 101(1–2):10–14
Funding
This study was financially supported by the “Leading Goose” R&D Program of Zhejiang (No. 2022C03050), the China National Key R&D Program (No. 2017YFE0130100), the Science and Technology Planning Project of Jinhua (NO. 2020-1-025), the Jiaxing Key Discipiline of Medicine-Clinical Pharmacy (No. 2023-ZC-008) and the Science and Technology Planning Project of Quzhou (NO. 2021K23).
Author information
Authors and Affiliations
Contributions
PW and WS conceived and designed the experiments; SL and ZC performed the experiments; ZW, and FF analyzed and interpreted the data; ZX and YT contributed reagents and analysis tools; SL and ZC wrote the paper. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
The animal experiment part of this project was approved by the animal ethics committee of Zhejiang University of Technology with approval number 20190603064, and experiment was conducted as per the Guide for the Care and Use of Laboratory Animals at the Zhejiang University of Technology, Hangzhou, China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, S., Chen, Z., Wu, Z. et al. Involvement of PI3K/AKT Pathway in the Rapid Antidepressant Effects of Crocetin in Mice with Depression-Like Phenotypes. Neurochem Res 49, 477–491 (2024). https://doi.org/10.1007/s11064-023-04051-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04051-2