Abstract
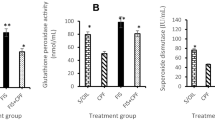
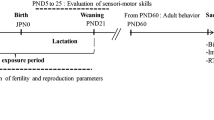
Carbendazim (CBZ) is one of the most common fungicides used to fight plant fungal diseases, otherwise, it leaves residue on fruits, vegetables, and soil that contaminate the environment, water, animal, and human causing serious health problems. Several studies have reported the reproductive and endocrine pathological disorders induced by CBZ in several animal models, but little is known about its neurotoxicity. So that, the present study aimed to explain the possible mechanisms of CBZ induced neurotoxicity in rats. Sixty male Wistar rats were divided into 4 groups (n = 15). Group (1) received normal saline and was kept as the negative control group, whereas groups (2, 3, 4) received CBZ at 100, 300, 600 mg/kg b.wt respectively. All rats received the aforementioned materials daily via oral gavage. Brain tissue samples were collected at 7, 14, 28 days from the beginning of the experiment. CBZ induced oxidative stress damage manifested by increasing MDA levels and reducing the levels of TAC, GSH, CAT in some brain areas at 14 and 28 days. There were extensive neuropathological alterations in the cerebrum, hippocampus, and cerebellum with strong caspase-3, iNOS, Cox-2 protein expressions mainly in rats receiving 600 mg/kg CBZ at each time point. Moreover, upregulation of mRNA levels of NF-κB, TNF-α, IL-1B genes and downregulation of the transcript levels of both AchE and MAO genes were recorded in all CBZ receiving groups at 14 and 28 days especially those receiving 600 mg/kg CBZ. Our results concluded that CBZ induced dose- and time-dependent neurotoxicity via disturbance of oxidant/antioxidant balance and activation of NF-κB signaling pathway. We recommend reducing the uses of CBZ in agricultural and veterinary fields or finding other novel formulations to reduce its toxicity on non-target organisms and enhance its efficacy on the target organisms.






Similar content being viewed by others
Data Availability
All data are available on request.
Abbreviations
- AchE:
-
Acetylcholinesterase
- ACTB:
-
Beta-actin housekeeping gene
- ATP:
-
Adenosine triphosphate
- b.wt:
-
Body weight
- CA:
-
Cornu Ammonis
- CAT:
-
Catalase
- CBZ:
-
Carbendazim
- Cox-2:
-
Cyclooxygenase-2
- DNA:
-
Deoxyribonucleic acid
- EDTA:
-
Ethylene diamine tetra acstic acid
- GSH:
-
Glutathione
- GTP:
-
Guanosine triphosphate
- H&E:
-
Hematoxylin & Eosin
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- LAF:
-
Lymphocyte-activating factor
- LD50:
-
Lethal dose-50
- MAO:
-
Monoamine oxidase
- MAPK:
-
Mitogen-activated protein kinase
- MDA:
-
Malondialdehyde
- MMP:
-
Matrix metalloproteinase
- mRNA:
-
Messenger ribonucleic acid
- NF-κB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- PBS:
-
Phosphate buffer saline
- PGE2:
-
Prostaglandine E2
- ROS:
-
Reactive oxygen species
- Rt-PCR:
-
Real-time polymerase chain reaction
- SEM:
-
Standard error of mean
- TAC:
-
Total antioxidant capacity
- TNF-α:
-
Tumor necrosis factor-alpha
References
Savary S, Ficke A, Aubertot JN, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Secur 4:519–537. https://doi.org/10.1007/s00203-017-1426-6
Karlsson I, Friberg H, Steinberg C, Persson P (2014) Fungicide effects on fungal community composition in the wheat phyllosphere. PLoS ONE 9:e111786. https://doi.org/10.1371/journal.pone.0111786
Patel GM, Rohit J, Singhal RK, Kailasa SK (2015) Recognition of carbendazim fungicide in environmental samples by using4-aminobenzenethiol functionalized silver nanoparticles as a colorimetric sensor. Sens Actuators B Chem 206:684–691. https://doi.org/10.1016/j.snb.2014.09.095
De A, Bose R, Kumar A, Mozumdar S (2014) Worldwide pesticide use. In: Targeted delivery of pesticides using biodegradable polymeric nanoparticles. Springer, New Delhi
Devi PA, Paramasivam M, Prakasam V (2015) Degradation pattern and risk assessment of carbendazim and mancozeb in mango fruits. Environ Monit Assess 187:1–6. https://doi.org/10.1007/s10661-014-4142-6
Selmanoglu G, Barlas N, Songur S, KocSkaya EA (2001) Carbendazim induced heamatological, biochemical and histopathological changes to the liver and kidney of male rats. Hum Exp Toxicol 20:625–630. https://doi.org/10.1191/096032701718890603
Bhushan C, Bhardwaj A, Misra SS (2013) State of pesticide regulations in India. Centre for Science and Environment, New Delhi
Banyiova K, Necasova A, Kohoutek J, Justan I, Čupr P (2016) New experimental data on the human dermal absorption of simazine and carbendazim help to refine the assessment of human exposure. Chemosphere 145:148–156. https://doi.org/10.1016/j.chemosphere.2015.11.018
Goodson WH, Lowe L, Carpenter DO et al (2015) Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcin 36:254–296. https://doi.org/10.1093/carcin/bgv039
Ferreira AL, Loureiro S, Soares AM (2008) Toxicity prediction of binary combinations of cadmium, carbendazim and low dissolved oxygen on Daphnia magna. Aqua Toxicol 89:28–39. https://doi.org/10.1016/j.aquatox.2008.05.012
Zhang X, Huang Y, Harvey PR, Li H, Ren Y (2013) Isolation and characterization of carbendazim-degrading Rhodococcus erythropolis djl-11. PLoS ONE 8:1–6. https://doi.org/10.1371/journal.pone.0074810
Huan Z, Luo J, Xu Z, Xie D (2016) Acute toxicity and genotoxicity of carbendazim, main impurities and metabolite to earthworms (Eisenia foetida). Bull Environ Contam Toxicol 96:62–69. https://doi.org/10.1007/s00128-015-1653-y
Singh S, Singh N, Kumar V, Datta S, Wani A, Singh D, Singh K, Singh J (2016) Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ Chem Lett 14:317–329. https://doi.org/10.1007/s10311-016-0566-2
Morinaga H, Yanase T, Nomura M, Okabe T, Goto K, Harada N, Nawata H (2004) A benzimidazole fungicide, benomyl, and its metabolite, carbendazim, induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN). Endocrinol 145:1860–1869. https://doi.org/10.1210/en.2003-1182
Yu Y, Chu X, Pang G, Xiang Y, Fang H (2009) Effects of repeated applications of fungicide carbendazim on its persistence and microbial community in soil. J Environ Sci 21(2):179–185. https://doi.org/10.1016/S1001-0742(08)62248-2
Winder BS, Strandgaard CS, Miller MG (2001) The role of GTP binding and microtubule-associated proteins in the inhibition of microtubule assembly by carbendazim. Toxicol Sci 59:138–146. https://doi.org/10.1093/toxsci/59.1.138
Rama EM, Bortolan S, Vieira ML, Gerardin DC, Moreira EG (2014) Reproductive and possible hormonal effects of carbendazim. Regul Toxicol Pharmacol 69:476–486. https://doi.org/10.1016/j.yrtph.2014.05.016
Salihu M, Ajayi BO, Adedara IA, Farombi EO (2015) 6-Gingerol-rich fraction from Zingiber Officinale prevents hematotoxicity and oxidative damage in kidney and liver of rats exposed to carbendazim. J Diet Suppl 16:1–16. https://doi.org/10.3109/19390211.2015.1107802
Zubrod JP, Baudy P, Schulz R, Bundschuh M (2014) Effects of current-use fungicides and their mixtures on the feeding and survival of the key shredder Gammarus fossarum. Aquat Toxicol 150:133–143. https://doi.org/10.1016/j.aquatox.2014.03.002
Hsu YH, Chang CW, Chen MC, Yuan CY (2011) Carbendazim induced androgen receptor expression antagonized by flutamide in male rats. J Food Drug Anal 19:4
Yenjerla M, Cox C, Wilson L, Jordan MA (2009) Carbendazim inhibits cancer cell proliferation by suppressing microtubule dynamics. J Pharmacol Exp Ther 328:390–398. https://doi.org/10.1124/jpet.108.143537
Abdel-Mobdy YE, Saleh MA, Nassar DM, Kandil MA (2018) Subchronic effect of carbendazim on spermatogenesis and fertility in male albino rats before and after accelerated storage. Res J Pharm Biol Chem Sci 9(5):303
Nowzo SO, Ozegbe PC, Olasehinde O (2017) Carbendazim alters kidney morphology, kidney function tests, tissue markers of oxidative stress and serum micro-elements in rats fed protein-energy malnourished diet. Int J Biol Chem Sci 11(3):1046–1055
Owumi SE, Nowozo SO, Najophe ES (2019) Quercetin abates induction of hepatic and renal oxidative damage, inflammation, and apoptosis in carbendazim-treated rats. Toxicol Res Appl 3:1–8. https://doi.org/10.1177/2397847319849521
Jin Y, Zeng Z, Wu Y, Zhang S, Fu Z (2015) Oral exposure of mice to carbendazim induces hepatic lipid metabolism disorder and gut microbiota dysbiosis. Toxicol Sci 147(1):116–126
Frye CA, Walf AA (2002) Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrus rats. Horm Behav 41(3):306–315. https://doi.org/10.1006/hbeh.2002.1763
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–328. https://doi.org/10.1038/nprot.2007.44
Rasoulijazi H, Joghataei M, Noubakht M, Roughani M (2007) The beneficial effect of (-)-epigallocatechin-3-gallate in an experimental model of Alzheimer disease in rat: a behavioral analysis. Iran Biomed J 11(4):237–243
Bancroft JD, Layton C (2013) The hematoxylins and eosin. In: Suvarna Kim (ed) Bancroft’s theory and practice of histological techniques. Churchill Living tone, London, pp 173–186
Gad SC, Rousseaux CG (2002) Use and misuse of statistics as an aid in study interpretation. In: Haschek WM, Rousseaux CG, Wallig MA (eds) Handbook of toxicologic pathology. Academic Press, San Diego, pp 327–418
Chaâbane M, Ghorbel I, Elwej A, Mnif H, Boudawara T, Chaâbouni SE, Zeghal N, Soudani N (2017) Penconazole alters redox status, cholinergic function, and membrane bound ATPases in the cerebrum and cerebellum of adult rats. Hum Exp Toxicol 36(8):1–13
Hassanen EI, Ibrahim MA, Hassan AM, Mehanna S, Aljuaydi SH, Issa MY (2021) Neuropathological and cognitive effects induced by CuO–NPs in rats and trials for prevention using pomegranate juice. Neurochem Res 46:1264–1279. https://doi.org/10.1007/s11064-021-03264-7
Derelanko MJ (2000) Acute/chronic toxicology. In: Toxicologist’s pocket handbook. CRC Press, Boca Raton
Hassanen EI, Korany RMS, Bakeer AM (2021) Cisplatin conjugated gold nanoparticles-based drug delivery system for targeting hepatic tumors. J Biochem Mol Toxicol 35(5):e22722. https://doi.org/10.1002/jbt.22722
Jiang JH, Wu SG, Wu CX, An XH, Cai LM (2014) Embryonic exposure to carbendazim induces the transcription of genes related to apoptosis, immunotoxicity and endocrine disruption in zebrafish (Daniorerio). Fish Shellfish Immunol 41:493–500
Apaydin M, Erbas O, Taskiran D (2016) Protection by edaravone, a radical scavenger, against manganese-induced neurotoxicity in rats. J Biochem Mol Toxicol 30:5
Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD (2012) The mouse forced swim test. J Vis Exp 59:e3638. https://doi.org/10.3791/3638
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behaviour in rodents. Nat Protoc 2(2):322–328. https://doi.org/10.1038/nprot.2007.44
Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 1916:105–111. https://doi.org/10.1007/978-1-4939-8994-2_10
Mufson EJ, Counts SE, Perez SE, Ginsberg SD (2008) Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother 8:1703–1718
Kim D, Baik SH, Kang S, Cho SW, Bae J, Cha MY, Sailor MJ, Mook-Jung I, Ahn KH (2016) Close correlation of monoamine oxidase activity with progress of Alzheimer’s disease in mice. Observed by in vivo two-photon imaging. ACS Cent Sci 2(12):967–975. https://doi.org/10.1021/acscentsci.6b00309
Adedara IA, Vaithinathan S, Jubendradass R, Mathur PP, Farombi EO (2013) Kolavir on prevents carbendazim-induced steroidogenic dysfunction and apoptosis in testes of rats. Environ Toxicol Pharmacol 35:444–453. https://doi.org/10.1016/j.etap.2013.01.010
Lutz P (2012) Benzimidazole and its derivatives—from fungicides to designer drug. A new occupational and environmental hazard. Med Pr 63(4):505–513
Dikić D, Landeka I, Knežević F, Mojsović- Ćuić A, Benković V, Horvat-Knežević A, Lončar G, Teparić R, Rogić D (2012) Carbendazim impends hepatic necrosis when combined with imazalil or cypermethrin. Basic Clin Pharmacol Toxicol 110(5):433–440. https://doi.org/10.1111/j.1742-7843.2011.00831.x
McDaniel KL, Padilla S, Marshall RS, Phillips PM, Podhorniak L, Qian Y, Moser VC (2007) Comparison of acute neurobehavioral and cholinesterase inhibitory effects of N-methylcarbamates in rat. Toxicol Sci 98(2):552–560
Yeung PK, Lai AK, Son HJ, Zhang X, Hwang O, Chung SS, Chung SK (2017) Aldose reductase deficiency leads to oxidative stress-induced dopaminergic neuronal loss and autophagic abnormality in an animal model of Parkinson’s disease. Neurobiol Aging 50:119–133
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10:49
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases. Int J Physiol Pathophysiol Pharmacol 87:245–313
Ahmad R, Rasheed Z, Ahsan H (2009) Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacol Immunotoxicol 31:388–396
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Nicholls DG (2002) Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Biochem Cell Biol 34:1372–1381
Zhao Y, Newman MC (2006) Effects of exposure duration and recovery time during pulsed exposures. Environ Toxicol Chem 25:1298–1304
Damalas CA, Koutroubas SD (2016) Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxics 4:1. https://doi.org/10.3390/toxics4010001
Fenske RA, Day EW (2005) Assessment of exposure for pesticide handlers in agricultural, residential and institutional environments. In: Occupational and residential exposure assessment for pesticides. Wiley, Chichester, pp 13–43. https://doi.org/10.1002/0470012218.CH1
Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D (2009) Cyclooxygenase in normal human tissues—is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med 13:3753–3763
Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S (2003) Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci USA 100(9):5473–5478. https://doi.org/10.1073/pnas.0837397100
Wang M, Gorasiya S, Antoine DJ, Sitapara RA, Wu W, Sharma L et al (2015) The compromise of macrophage functions by hyperoxia is attenuated by ethacrynic acid via inhibition of NF-kappaB-mediated release of high-mobility group box-1. Am J Respir Cell Mol 52(2):171–182. https://doi.org/10.1165/rcmb.2013-0544OC
Im JY, Kim D, Paik SG, Han PL (2006) Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Rad Biol Med 41:960–972. https://doi.org/10.1016/j.freeradbiomed.2006.06.001
Banks CN, Lein JA (2012) Review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicol 33:575–584
Chambers J, Oppenheimer SF (2004) Organophosphates, serine esterase inhibition, and modeling of organophosphate toxicity. Toxicol Sci 77:185–187
Cao DL, Zhang ZJ, Xie RG, Jiang BC, Ji RR, Gao YJ (2014) Chemokine CXCL1enhances inflammatory pain and increases NMDA receptor activity andCOX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol 261:328–336
Ogata S, Kubota Y, Yamashiro T, Takeuchi H, Ninomiya T, Suyama Y, Shirasuna K (2007) Signaling pathways regulating IL-1alpha-induced COX-2 expression. J Dent Res 86:186–191
Zeng M, Tong QY (2020) Anti-inflammation effects of sinomenine on macrophages through suppressing activated tlr4/nf-kappa b signaling pathway. Curr Med Sci 40(1):130–137
Sun G, Guzman E, Balasanyan V, Conner CM, Wong K, Zhou HR, Kosik KS, Montell DJ (2017) A molecular signature for anastasis, recovery from the brink of apoptotic cell death. J Cell Biol 16(10):3355–3368. https://doi.org/10.1083/jcb.201706134
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
Garlanda C, Dinarello CA, Mantovani A (2013) The interleukin-1 family: back to the future. Immunity 39:1003–1018. https://doi.org/10.1016/j.immuni.2013.11.010
Hussain SP, Hofseth LJ, Harris CC (2003) Radical causes of cancer. Nat Rev Cancer 3:276–285
Fichera LE, Albareda MC, Laucella SA, Postan M (2004) Intracellular growth of Trypanosoma cruzi in cardiac myocytes is inhibited by cytokine-induced nitric oxide release. Infect Immun 72:359–363
Teixeira CC, Ischiropoulos H, Leboy PS, Adams SL, Shapiro IM (2005) Nitric oxide-nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone 37:37–45
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424
Lossi L, Castagna C, Merighi A (2015) Neuronal cell death: an overview of its different forms in central and peripheral neurons. In: Neuronal cell death. Springer, New York, pp 1–18
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5:a008656
Khalaf AA, Hassanen EI, Ibrahim MA, Tohamy AF, Aboseada MA, Hassan HM, Zaki AR (2020) Rosmarinic acid attenuates chromium-induced hepatic and renal oxidative damage and DNA damage in rats. J Biochem Mol Toxicol 34(11):e22579
Choudhary GS, Al-Harbi S, Almasan A (2015) Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 1219:1–9
Hassanen EI, Khalaf AA, Tohamy AF, Mohammed ER, Farroh KY (2019) Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int J Nanomed 14:4723–4739. https://doi.org/10.2147/IJN.S207644
Hassanen EI, Tohamy AF, Hassan AM, Ibrahim MA, Issa MY, Farroh KY (2019) Pomegranate juice diminishes the mitochondrial-dependent cell death and NF-ĸB signaling pathway induced by Copper oxide nanoparticles on the liver and kidneys of rats. Int J Nanomed 14:8905–8922
Gheena S, Ezhilarasan D (2019) Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum Exp Toxicol 38:694–702
Almagro MC, Vucic D (2015) Necroptosis: pathway diversity and characteristics. Semin Cell Dev Biol 39(56):62
Funding
This research didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing interest declared.
Ethical Approval
All Institutional and National Guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebedy, Y.A., Hassanen, E.I., Hussien, A.M. et al. Neurobehavioral Toxicity Induced by Carbendazim in Rats and the Role of iNOS, Cox-2, and NF-κB Signalling Pathway. Neurochem Res 47, 1956–1971 (2022). https://doi.org/10.1007/s11064-022-03581-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03581-5