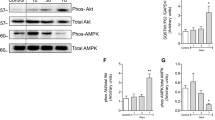
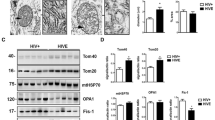
Abstract
Rabies is a fatal encephalitis caused by the Rabies lyssavirus (RABV). The presence of minimal neuropathological changes observed in rabies indicates that neuronal dysfunction, rather than neuronal death contributes to the fatal outcome. The role of mitochondrial changes has been suggested as a possible mechanism for neuronal dysfunction in rabies. However, these findings are mostly based on studies that have employed experimental models and laboratory-adapted virus. Studies on brain tissues from naturally infected human and animal hosts are lacking. The current study investigated the role of mitochondrial changes in rabies by morphological, biochemical and proteomic analysis of RABV-infected human and canine brains. Morphological analysis showed minimal inflammation with preserved neuronal and disrupted mitochondrial structure in both human and canine brains. Proteomic analysis revealed involvement of mitochondrial processes (oxidative phosphorylation, cristae formation, homeostasis and transport), synaptic proteins and autophagic pathways, with over-expression of subunits of mitochondrial respiratory complexes. Consistent with these findings, human and canine brains displayed elevated activities of complexes I (p < 0.05), IV (p < 0.05) and V (p < 0.05). However, this did not result in elevated ATP production (p < 0.0001), probably due to lowered mitochondrial membrane potential as noted in RABV-infected cells in culture. These could lead to mitochondrial dysfunction and mitophagy as indicated by expression of FKBP8 (p < 0.05) and PINK1 (p < 0.001)/PARKIN (p > 0.05) and ensuing autophagy, as shown by the status of LCIII (p < 0.05), LAMP1 (p < 0.001) and pertinent ultrastructural markers. We propose that altered mitochondrial bioenergetics and cristae architecture probably induce mitophagy, leading to autophagy and consequent neuronal dysfunction in rabies.









Similar content being viewed by others
Data Availability
The MS data presented in the current manuscript have been deposited in the ProteomeXchange Consortium via the PRIDE partner repository [79] with the dataset identifier PXD020789 (http://www.ebi.ac.uk/pride).
Abbreviations
- CI:
-
Complex I
- CII:
-
Complex II
- CIII:
-
Complex III
- CIV:
-
Complex IV
- CV:
-
Complex V
- CVS:
-
Challenge Virus Standard
- EM:
-
Electron microscopy
- HE:
-
Haematoxylin and eosin
- MS:
-
Mass spectrometry
- RABV:
-
Rabies lyssavirus
- ROS:
-
Reactive oxygen species
- RRID:
-
Research resource identifier
- SV:
-
Street virus
- TMT:
-
Tandem mass tag
References
Hampson K, Coudeville L, Lembo T et al (2015) Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 9:e0003709. https://doi.org/10.1371/Journal.PNTD.0003709
WHO expert consultation on rabies (2018): third report. World Health Organization
Murphy FA (1977) Rabies pathogenesis brief review. Arch Virol 54:279–297
Tsiang H (1982) Neuronal function impairment in rabies-infected rat brain. J Gen Virol 61:277–281. https://doi.org/10.1099/0022-1317-61-2-277
Rossiter JP, Jackson AC (2013) Chapter 9—pathology. In: Jackson AC (ed) Rabies, 3rd edn. Academic Press, Boston, pp 351–386
Scott CA, Rossiter JP, Andrew RD, Jackson AC (2008) Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol 82:513–521. https://doi.org/10.1128/jvi.01677-07
Song Y, Hou J, Qiao B et al (2013) Street rabies virus causes dendritic injury and F-actin depolymerization in the hippocampus. J Gen Virol 94:276–283. https://doi.org/10.1099/vir.0.047480-0
Li X-Q, Sarmento L, Fu ZF (2005) Degeneration of neuronal processes after infection with pathogenic, but not attenuated, rabies viruses. J Virol 79:10063–10068. https://doi.org/10.1128/jvi.79.15.10063-10068.2005
Jackson AC, Kammouni W, Zherebitskaya E, Fernyhough P (2010) Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons. J Virol 84:4697–4705. https://doi.org/10.1128/jvi.02654-09
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. https://doi.org/10.1038/nature05292
Claus C, Liebert UG (2014) A renewed focus on the interplay between viruses and mitochondrial metabolism. Arch Virol 159:1267–1277
Alandijany T, Kammouni W, Roy Chowdhury SK et al (2013) Mitochondrial dysfunction in rabies virus infection of neurons. J Neurovirol 19:537–549. https://doi.org/10.1007/s13365-013-0214-6
Jackson AC, Kammouni W, Fernyhough P (2011) Role of oxidative stress in rabies virus infection. Adv Virus Res 79:127–138. https://doi.org/10.1016/B978-0-12-387040-7.00008-1
Kammouni W, Wood H, Jackson AC (2017) Lyssavirus phosphoproteins increase mitochondrial complex I activity and levels of reactive oxygen species. J Neurovirol 23:756–762. https://doi.org/10.1007/s13365-017-0550-z
Kammouni W, Wood H, Saleh A et al (2015) Rabies virus phosphoprotein interacts with mitochondrial Complex I and induces mitochondrial dysfunction and oxidative stress. J Neurovirol 21:370–382. https://doi.org/10.1007/s13365-015-0320-8
Venugopal AK, Ghantasala SSK, Selvan LDN et al (2013) Quantitative proteomics for identifying biomarkers for rabies. Clin Proteomics 10:1. https://doi.org/10.1186/1559-0275-10-3
Woldehiwet Z (2002) Rabies: recent developments. Res Vet Sci 73:17–25. https://doi.org/10.1016/S0034-5288(02)00046-2
Harish G, Mahadevan A, Pruthi N et al (2015) Characterization of traumatic brain injury in human brains reveals distinct cellular and molecular changes in contusion and pericontusion. J Neurochem 134:156–172. https://doi.org/10.1111/jnc.13082
Harish G, Venkateshappa C, Mahadevan A et al (2012) Effect of premortem and postmortem factors on the distribution and preservation of antioxidant activities in the cytosol and synaptosomes of human brains. Biopreserv Biobank 10:253–265. https://doi.org/10.1089/bio.2012.0001
Karunakaran S, Saeed U, Ramakrishnan S et al (2007) Constitutive expression and functional characterization of mitochondrial glutaredoxin (Grx2) in mouse and human brain. Brain Res 1185:8–17. https://doi.org/10.1016/j.brainres.2007.09.019
Karunakaran S, Saeed U, Mishra M et al (2008) Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. J Neurosci 28:12500–12509. https://doi.org/10.1523/JNEUROSCI.4511-08.2008
Harish G, Venkateshappa C, Mahadevan A et al (2013) Mitochondrial function in human brains is affected by pre- and post mortem factors. Neuropathol Appl Neurobiol 39:298–315. https://doi.org/10.1111/j.1365-2990.2012.01285.x
Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK (2013) Alteration in glutathione content and associated enzyme activities in the synaptic terminals but not in the non-synaptic mitochondria from the frontal cortex of Parkinson’s disease brains. Neurochem Res 38:186–200. https://doi.org/10.1007/s11064-012-0907-x
Venkateshappa C, Harish G, Mahadevan A et al (2012) Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer’s disease. Neurochem Res 37:1601–1614. https://doi.org/10.1007/s11064-012-0755-8
Venkateshappa C, Harish G, Mythri RB et al (2012) Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson’s disease. Neurochem Res 37:358–369. https://doi.org/10.1007/s11064-011-0619-7
Mythri RB, Venkateshappa C, Harish G et al (2011) Evaluation of Markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res 36:1452–1463. https://doi.org/10.1007/s11064-011-0471-9
Alladi PA, Mahadevan A, Yasha TC et al (2009) Absence of age-related changes in nigral dopaminergic neurons of Asian Indians: relevance to lower incidence of Parkinson’s disease. Neuroscience 159:236–245. https://doi.org/10.1016/j.neuroscience.2008.11.051
Chandana R, Mythri RB, Mahadevan A et al (2009) Biochemical analysis of protein stability in human brain collected at different post-mortem intervals. Indian J Med Res 129:189–199
Harish G, Venkateshappa C, Mahadevan A et al (2011) Effect of storage time, postmortem interval, agonal state, and genderon the postmortem preservation of glial fibrillary acidic protein and oxidatively damaged proteins in human brains. Biopreserv Biobank 9:379–387. https://doi.org/10.1089/bio.2011.0033
Harish G, Venkateshappa C, Mahadevan A et al (2011) Glutathione metabolism is modulated by postmortem interval, gender difference and agonal state in postmortem human brains. Neurochem Int 59:1029–1042. https://doi.org/10.1016/j.neuint.2011.08.024
Chinta SJ, Kommaddi RP, Turman CM et al (2005) Constitutive expression and localization of cytochrome P-450 1A1 in rat and human brain: presence of a splice variant form in human brain. J Neurochem 93:724–736. https://doi.org/10.1111/j.1471-4159.2005.03061.x
Agarwal V, Kommaddi RP, Valli K et al (2008) Drug metabolism in human brain: high levels of cytochrome P4503A43 in brain and metabolism of anti-anxiety drug alprazolam to its active metabolite. PLoS ONE 3:e0002337. https://doi.org/10.1371/journal.pone.0002337
Alladi PA, Mahadevan A, Shankar SK et al (2010) Expression of GDNF receptors GFRα1 and RET is preserved in substantia nigra pars compacta of aging Asian Indians. J Chem Neuroanat 40:43–52. https://doi.org/10.1016/j.jchemneu.2010.03.007
Alladi PA, Mahadevan A, Vijayalakshmi K et al (2010) Ageing enhances α-synuclein, ubiquitin and endoplasmic reticular stress protein expression in the nigral neurons of Asian Indians. Neurochem Int 57:530–539. https://doi.org/10.1016/j.neuint.2010.06.018
Dean DJ (1966) Laboratory techniques in rabies. The fluorescent antibody test. Monogr Ser World Health Organ 23:59–68
Sheehan D (1987) Theory and practice of histotechnology, 2nd edn. Battelle Press, Columbus
Frasca JM, Parks VR (1965) A routine technique for double-staining ultrathin sections using uranyl and lead salts. J Cell Biol 25:157–161. https://doi.org/10.1083/jcb.25.1.157
Hayat MA (2000) Principles and techniques of electron microscopy: biological applications, 4th edn. Cambridge University Press, Cambridge, p 543
Trounce IA, Kim YL, Jun AS, Wallace DC (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509. https://doi.org/10.1016/s0076-6879(96)64044-0
Wang W, Yang X, De Silanes IL et al (2003) Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem 278:27016–27023. https://doi.org/10.1074/jbc.M300318200
Bradford M (1976) A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Sharma T, Datta KK, Kumar M et al (2020) Intracranial aneurysm biomarker candidates identified by a proteome-wide study. Omi A J Integr Biol 24:483–492. https://doi.org/10.1089/omi.2020.0057
Kumar M, Varun CN, Dey G et al (2018) Identification of host-response in cerebral malaria patients using quantitative proteomic analysis. Proteomics 12:1600187. https://doi.org/10.1002/prca.201600187
Farahtaj F, Zandi F, Khalaj V et al (2013) Proteomics analysis of human brain tissue infected by street rabies virus. Mol Biol Rep 40:6443–6450. https://doi.org/10.1007/s11033-013-2759-0
Li L, Jin H, Wang H et al (2017) Autophagy is highly targeted among host comparative proteomes during infection with different virulent RABV strains. Oncotarget 8:21336–21350. https://doi.org/10.18632/oncotarget.15184
Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25:1091–1093
Calvo SE, Clauser KR, Mootha VK (2016) MitoCarta: an inventory of mammalian mitochondrial genes|Broad Institute. Nucleic acids Res 44:D1251–D1257
Pagliarini DJ, Calvo SE, Chang B et al (2008) A mitochondrial protein compendium elucidates complex I disease Biology. Cell 134:112–123. https://doi.org/10.1016/j.cell.2008.06.016
Zhang W, Zhang Y, Zheng H, Zhang C, Xiong W, OlyarchukWalker JGM (2007) SynDB: a synapse protein DataBase based on synapse ontology. Nucleic Acids Res 35:D737–D741
Renjini R, Gayathri N, Nalini A, Srinivas Bharath MM (2012) Analysis of calpain-3 protein in muscle biopsies of different muscular dystrophies from India. Indian J Med Res 135:878–886
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Tyanova S, Temu T, Sinitcyn P et al (2016) The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 13:731–740
Peng J, Zhu S, Hu L et al (2016) Wild-type rabies virus induces autophagy in human and mouse neuroblastoma cell lines. Autophagy 12:1704–1720. https://doi.org/10.1080/15548627.2016.1196315
Fooks AR, Cliquet F, Finke S et al (2017) Rabies. Nat Rev Dis Prim 3:17091. https://doi.org/10.1038/nrdp.2017.91
Mahadevan A, Suja MS, Mani RS, Shankar SK (2016) Perspectives in diagnosis and treatment of rabies viral encephalitis: insights from pathogenesis. Neurotherapeutics 13:477–492
Piccinotti S, Whelan SPJ (2016) Rabies internalizes into primary peripheral neurons via clathrin coated pits and requires fusion at the cell body. PLoS Pathog 12:1–25. https://doi.org/10.1371/journal.ppat.1005753
Piccinotti S, Kirchhausen T, Whelan SPJ (2013) Uptake of rabies virus into epithelial cells by clathrin-mediated endocytosis depends upon actin. J Virol 87:11637–11647. https://doi.org/10.1128/jvi.01648-13
Walsh D, Naghavi MH (2019) Exploitation of cytoskeletal networks during early viral infection. Trends Microbiol 27:39–50
Dräger NM, Nachman E, Winterhoff M et al (2017) Bin1 directly remodels actin dynamics through its BAR domain. EMBO Rep 18:2051–2066. https://doi.org/10.15252/embr.201744137
Takenawa T, Suetsugu S (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8:37–48. https://doi.org/10.1038/nrm2069
Correas I, Padilla R, Avila J (1990) The tubulin-binding sequence of brain microtubule-associated proteins, tau and MAP-2, is also involved in actin binding. Biochem J 269:61–64. https://doi.org/10.1042/bj2690061
Cogliati S, Enriquez JA, Scorrano L (2016) Mitochondrial cristae: where beauty meets functionality. Trends Biochem Sci 41:261–273
Guarani V, McNeill EM, Paulo JA et al (2015) QIL1 is a novel mitochondrial protein required for MICOS complex stability and cristae morphology. Elife 4:1–23. https://doi.org/10.7554/eLife.06265
Xie J, Marusich MF, Souda P et al (2007) The mitochondrial inner membrane protein Mitofilin exists as a complex with SAM50, metaxins 1 and 2, coiled-coil-helix coiled-coil-helix domain-containing protein 3 and 6 and DnaJC11. FEBS Lett 581:3545–3549. https://doi.org/10.1016/j.febslet.2007.06.052
Harner M, Körner C, Walther D et al (2011) The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J 30:4356–4370. https://doi.org/10.1038/emboj.2011.379
Head BP, Zulaika M, Ryazantsev S, Van Der Bliek AM (2011) A novel mitochondrial outer membrane protein, MOMA-1, that affects cristae morphology in Caenorhabditis elegans. Mol Biol Cell 22:831–841. https://doi.org/10.1091/mbc.E10-07-0600
Weber TA, Koob S, Heide H et al (2013) APOOL is a cardiolipin-binding constituent of the mitofilin/MINOS protein complex determining cristae morphology in mammalian mitochondria. PLoS ONE 8:e63683. https://doi.org/10.1371/journal.pone.0063683
Davies KM, Anselmi C, Wittig I et al (2012) Structure of the yeast F 1F o-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc Natl Acad Sci USA 109:13602–13607. https://doi.org/10.1073/pnas.1204593109
Eydt K, Davies KM, Behrendt C et al (2017) Cristae architecture is determined by an interplay of the MICOS complex and the F1Fo ATP synthase via Mic27 and Mic10. Microb Cell 4:259–272. https://doi.org/10.15698/mic2017.08.585
Strauss M, Hofhaus G, Schröder RR, Kühlbrandt W (2008) Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J 27:1154–1160. https://doi.org/10.1038/emboj.2008.35
Ramadasan-Nair R, Gayathri N, Mishra S et al (2014) Mitochondrial alterations and oxidative stress in an acute transient mouse model of muscle degeneration: implications for muscular dystrophy and related muscle pathologies. J Biol Chem 289:485–509. https://doi.org/10.1074/jbc.M113.493270
Sunitha B, Gayathri N, Kumar M et al (2016) Muscle biopsies from human muscle diseases with myopathic pathology reveal common alterations in mitochondrial function. J Neurochem 138:174–191. https://doi.org/10.1111/jnc.13626
Liu J, Wang H, Gu J et al (2017) BECN1-dependent CASP2 incomplete autophagy induction by binding to rabies virus phosphoprotein. Autophagy 13:739–753. https://doi.org/10.1080/15548627.2017.1280220
Sun Y, Yu S, Ding N et al (2014) Autophagy benefits the replication of newcastle disease virus in chicken cells and tissues. J Virol 88:525–537. https://doi.org/10.1128/jvi.01849-13
Heaton NS, Randall G (2011) Dengue virus and autophagy. Viruses 3:1332–1341
Jackson AC (2000) Rabies. Can J Neurol Sci 27(4):278–282
Beck S, Gunawardena P, Horton DL et al (2017) Pathobiological investigation of naturally infected canine rabies cases from Sri Lanka. BMC Vet Res 13:1–9. https://doi.org/10.1186/s12917-017-1024-5
Farahtaj F, Alizadeh L, Gholami A et al (2019) Natural infection with rabies virus: a histopathological and immunohistochemical study of human brains. Osong Public Heal Res Perspect 10:6–11. https://doi.org/10.24171/j.phrp.2019.10.1.03
Perez-Riverol Y, Csordas A, Bai J et al (2019) The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47:D442–D450. https://doi.org/10.1093/nar/gky1106
Acknowledgements
The technical help provided by Mr. Ramesh, Ms. Rashmi S and Mr. Satheesh from the Electron Microscopy Facility, Department of Neuropathology, NIMHANS is gratefully acknowledged. The assistance of Mr. Shivaji Rao and Mrs. Rajasakti from the Human Brain Tissue Repository, NIMHANS with the histopathology experiments is gratefully acknowledged. The authors acknowledge the help of Dr. Gajanan Sathe, Institute of Bioinformatics, Bangalore for his help with analysis of MS data.
Funding
PKH is supported through the project titled “Testing of blood samples for rabies virus neutralizing antibodies by RFFIT” (Grant no. OTHERS/001/107/2014/00713), at the department of Neurovirology, NIMHANS, Bangalore, India, funded by Cadila Pharmaceuticals, India (Principal Investigator-RSM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
PKH: Methodology; data curation; validation; formal analysis; writing—original draft preparation, SR: conceptualization; data curation; writing—original draft preparation, GY: resources, KKM: methodology; data curation, GD: methodology; data curation, AY; methodology, BKCS: analysis, AM: conceptualization; data analysis; writing-review and editing; resources; supervision, MMSB: conceptualization; writing-review and editing, supervision; project administration, RSM: conceptualization; writing-review and editing, supervision; project administration; resources.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harsha, P.K., Ranganayaki, S., Yale, G. et al. Mitochondrial Dysfunction in Rabies Virus-Infected Human and Canine Brains. Neurochem Res 47, 1610–1636 (2022). https://doi.org/10.1007/s11064-022-03556-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03556-6