Abstract
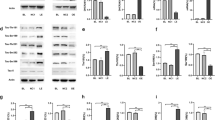
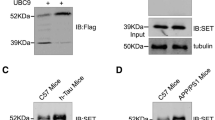
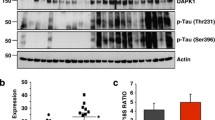
Accumulating data suggest that the downregulation of DHCR24 is linked to the pathological risk factors of AD, denoting a potential role of DHCR24 in AD pathogenesis. However, it remains unclear whether the downregulation of DHCR24 affects the abnormal heper-phosphorylation of tau protein, which is involved in tauopathy. In present papers, immunofluorescence and Filipin III fluorescence results showed that DHCR24 knockdown significantly lowered the level of plasma membrane cholesterol and expression level of membrane lipid-raft structural protein caveolin-1; and overexpression of DHCR24 could increase the plasma membrane cholesterol levels and facilitating caveolae structure through increase the expression of caveolin-1. PP2A is the key phosphatase involving in tau phosphorylation, which is localized in cholesterol-dependent caveola/raft lipid domains. Here, the PP2A activity was detected by western blot assay. Interestingly, the level of p-PP2Ac at Y307 (inactive) and p-GSK3β at Y216 (active) in the downstream of the PP2A signal pathway were both significantly increased in silencing DHCR24 SH-SY5Y cells, which denoted an inhibition of the PP2A and activation of GSK3β signaling. Conversely, overexpression of DHCR24 blunted the inhibition effect of PP2A and activation of GSK3β. Besides, in the SH-SY5Y cell lines we demonstrated that DHCR24 knockdown obviously induced hyperphosphorylation of tau at Thr181, Thr231, Ser262, Ser396, and Ser422 Sites. In contrast, DHCR24 overexpression protects neuronal SH-SY5Y cells against the hyperphosphorylation of tau at Thr181, Thr231, Ser262, Ser396, and Ser422 Sites. Furthermore, PP2A activator D-erythro-Sphingosine (DES) also obviously inhibited the hyperphosphorylation of tau induced by DHCR24 knockdown. Collectively, our findings firstly confirmed that DHCR24 knockdown obviously induced abnormal hyperphosphorylation of tau by a novel lipid raft-dependent PP2A signaling. We propose that DHCR24 downregulation led to altered cholesterol synthesis as a potential mechanism in the progression of tau hyperphosphorylation involving in AD and other tauopathies.








Similar content being viewed by others
References
Greeve I, Hermans-Borgmeyer I, Brellinger C, Kasper D, Gomez-Isla T, Behl C, Levkau B, Nitsch R (2000) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. J Neurosci 20(19):7345–7352
Zu H, Liu X, Yao K (2020) DHCR24 overexpression modulates microglia polarization and inflammatory response via Akt/GSK3β signaling in Aβ treated BV-2 cells. Life Sci 260:118470
Önmez A, Alpay M, Torun S, Şahin I, Öneç K (2020) Değirmenci Y (2020) Serum seladin-1 levels in diabetes mellitus and Alzheimer’s disease patients. Acta Neurol Belg 120(6):1399–1404
Kelicen-Ugur P, Cincioğlu-Palabıyık M, Çelik H, Karahan H (2019) Interactions of aromatase and seladin-1: a neurosteroidogenic and gender perspective. Transl Neurosci 10:264–279
Luciani P, Ferruzzi P, Arnaldi G, Crescioli C, Benvenuti S, Nesi G, Valeri A, Greeve I, Serio M, Mannelli M, Peri A (2004) Expression of the novel adrenocorticotropin-responsive gene selective Alzheimer’s disease indicator-1 in the normal adrenal cortex and in adrenocortical adenomas and carcinomas. J Clin Endocrinol Metab 89(3):1332–1339
Peri A (2016) Neuroprotective effects of estrogens: the role of cholesterol. J Endocrinol Investig 39(1):11–18
Zerenturk E, Sharpe L, Ikonen E, Brown A (2013) Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis. Prog Lipid Res 52(4):666–680
Zerenturk E, Kristiana I, Gill S (1821) Brown A (2012) The endogenous regulator 24(S),25-epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1). Biochim et Biophys Acta 9:1269–1277
Zhou C, Gao Z, Wang J, Wu M, Hu S, Chen F, Liu J, Pan H, Yan C (2018) Lead exposure induces Alzheimers’s disease (AD)-like pathology and disturbes cholesterol metabolism in the young rat brain. Toxicol Lett 296:173–183
Costa A, Joaquim H, Nunes V, Kerr D, Ferreira G, Forlenza O, Gattaz W, Talib L (2018) Donepezil effects on cholesterol and oxysterol plasma levels of Alzheimer’s disease patients. Eur Arch Psychiatry Clin Neurosci 268(5):501–507
Loera-Valencia R, Goikolea J, Parrado-Fernandez C, Merino-Serrais P, Maioli S (2019) Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J Steroid Biochem Mol Biol 190:104–114
Iivonen S, Hiltunen M, Alafuzoff I, Mannermaa A, Kerokoski P, Puoliväli J, Salminen A, Helisalmi S, Soininen H (2002) Seladin-1 transcription is linked to neuronal degeneration in Alzheimer’s disease. Neuroscience 113(2):301–310
Stock JB (2009) Protein phosphatase 2A: A novel pharmaceutical target for the treatment of Alzheimer’s disease. Alzheimers Dement 5(4):P319
Zhang H, Wang X, Xu P, Ji X, Chi T, Liu P, Zou L (2020) Tolfenamic acid inhibits GSK-3β and PP2A mediated tau hyperphosphorylation in Alzheimer’s disease models. J Physiol Sci 70(1):29
Sontag JM, Nunbhakdi-Craig V, Sontag E (2013) Leucine carboxyl methyltransferase 1 (LCMT1)-dependent methylation regulates the association of protein phosphatase 2A and Tau protein with plasma membrane microdomains in neuroblastoma cells. J Biol Chem 288(38):27396–27405
Xiong Y, Jing X, Zhou X, Wang X, Yang Y, Sun X, Qiu M, Cao F, Lu Y, Liu R, Wang J (2013) Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation. Neurobiol Aging 34(3):745–756
Buescher J, Phiel C (2010) A noncatalytic domain of glycogen synthase kinase-3 (GSK-3) is essential for activity. J Biol Chem 285(11):7957–7963
Zidovetzki R, Levitan I (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim et Biophys Acta 1768(6):1311–1324
Rao M, Peachman K, Alving C, Rothwell S (2003) Depletion of cellular cholesterol interferes with intracellular trafficking of liposome-encapsulated ovalbumin. Immunol Cell Biol 81(6):415–423
Biswas A, Kashyap P, Datta S, Sengupta T, Sinha B (2019) Cholesterol depletion by MβCD enhances cell membrane tension and its variations-reducing integrity. Biophys J 116(8):1456–1468
Krishna A, Sengupta D (2019) Interplay between membrane curvature and cholesterol: role of palmitoylated caveolin-1. Biophys J 116(1):69–78
Alonso A, Cohen L (2018) Our tau tales from normal to pathological behavior. J Alzheimer’s Dis 64:S507–S516
Iqbal K, Liu F, Gong C, Grundke-Iqbal I (2010) Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 7(8):656–664
Takashima A (2013) Tauopathies and tau oligomers. J Alzheimer’s Dis 37(3):565–568
Strang K, Golde T, Giasson B (2019) MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Investig 99(7):912–928
Saha P, Sen N (2019) Tauopathy: a common mechanism for neurodegeneration and brain aging. Mech Ageing Dev 178:72–79
Iqbal K, Liu F, Gong C, Alonso AC, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118(1):53–69
Gerson J, Mudher A, Kayed R (2016) Potential mechanisms and implications for the formation of tau oligomeric strains. Crit Rev Biochem Mol Biol 51(6):482–496
Alonso A, Beharry C, Corbo C, Cohen L (2016) Molecular mechanism of prion-like tau-induced neurodegeneration. Alzheimer’s Dement 12(10):1090–1097
Mudher A, Colin M, Dujardin S, Medina M, Dewachter I, Alavi Naini S, Mandelkow E, Mandelkow E, Buée L, Goedert M, Brion J (2017) What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol Commun 5(1):99
Di J, Cohen L, Corbo C, Phillips G, El Idrissi A, Alonso A (2016) Abnormal tau induces cognitive impairment through two different mechanisms: synaptic dysfunction and neuronal loss. Sci Rep 6:20833
Luu W, Hart-Smith G, Sharpe L, Brown A (2015) The terminal enzymes of cholesterol synthesis, DHCR24 and DHCR7, interact physically and functionally. J Lipid Res 56(4):888–897
Lu X, Kambe F, Cao X, Yoshida T, Ohmori S, Murakami K, Kaji T, Ishii T, Zadworny D, Seo H (2006) DHCR24-knockout embryonic fibroblasts are susceptible to serum withdrawal-induced apoptosis because of dysfunction of caveolae and insulin-Akt-Bad signaling. Endocrinology 147(6):3123–3132
Korade Z, Kenworthy A (2008) Lipid rafts, cholesterol, and the brain. Neuropharmacology 55(8):1265–1273
Mesa-Herrera F, Taoro-González L, Valdés-Baizabal C, Diaz M, Marín R (2019) Lipid and lipid raft alteration in aging and neurodegenerative diseases: a window for the development of new biomarkers. Int J Mol Sci 20(15):3810
Crameri A, Biondi E, Kuehnle K, Lütjohann D, Thelen K, Perga S, Dotti C, Nitsch R, Ledesma M, Mohajeri M (2006) The role of seladin-1/DHCR24 in cholesterol biosynthesis, APP processing and Abeta generation in vivo. EMBO J 25(2):432–443
Peri A, Serio M (2008) Neuroprotective effects of the Alzheimer’s disease-related gene seladin-1. J Mol Endocrinol 41(5):251–261
Waterham H, Koster J, Romeijn G, Hennekam R, Vreken P, Andersson H, FitzPatrick D, Kelley R, Wanders R (2001) Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am J Hum Genet 69(4):685–694
Habbab W, Aoude I, Palangi F, Abdulla S, Ahmed T (2019) The anti-tumor agent sodium selenate decreases methylated PP2A, increases GSK3betaY216 phosphorylation, including tau disease epitopes and reduces neuronal excitability in SHSY-5Y neurons. Int J Mol Sci 20(4):844
Raina V, Gupta S, Yadav S, Surolia A (2013) Simvastatin induced neurite outgrowth unveils role of cell surface cholesterol and acetyl CoA carboxylase in SH-SY5Y cells. PLoS ONE 8(9):e74547
Wang PY, Liu P, Weng J, Sontag E, Anderson RG (2003) A cholesterol-regulated PP2A/HePTP complex with dual specificity ERK1/2 phosphatase activity. EMBO J 22(11):2658–2667
Dobrowsky R, Kamibayashi C, Mumby M, Hannun Y (1993) Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem 268(21):15523–15530
Martin L, Page G, Terro F (2011) Tau phosphorylation and neuronal apoptosis induced by the blockade of PP2A preferentially involve GSK3β. Neurochem Int 59(2):235–250
Landrieu I, Smet-Nocca C, Amniai L, Louis J, Wieruszeski J, Goris J, Janssens V, Lippens G (2011) Molecular implication of PP2A and Pin1 in the Alzheimer’s disease specific hyperphosphorylation of Tau. PLoS ONE 6(6):e21521
Martin L, Latypova X, Wilson C, Magnaudeix A, Perrin M, Terro F (2013) Tau protein phosphatases in Alzheimer’s disease: the leading role of PP2A. Ageing Res Rev 12(1):39–49
Qian W, Shi J, Yin X, Iqbal K, Grundke-Iqbal I, Gong C, Liu F (2010) PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J Alzheimer’s Dis 19(4):1221–1229
Acknowledgements
This Work was supported by research grants 201740209 from the Shanghai Health and Medical Development Foundation, and JSZK2015A05 from the Shanghai Jinshan District Key Medical Foundation, Shanghai, China.
Author information
Authors and Affiliations
Contributions
HZ and XB designed the study and wrote the manuscript. ZQ contributed to the experimental work about tau phosphorylation and analysis of PP2A Signaling in SH-SY5Y Cells. YZ and MZ contributed to the tansfection work of SH-SY5Y cells. YX, JZ, and KY provided their experimental assistance and TEM work. XB and ZQ contributed to analysis of data. All authors have read the manuscript and provided the input.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All authors have approved the final article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, Z., Zhang, Y., Yao, K. et al. DHCR24 Knockdown Lead to Hyperphosphorylation of Tau at Thr181, Thr231, Ser262, Ser396, and Ser422 Sites by Membrane Lipid-Raft Dependent PP2A Signaling in SH-SY5Y Cells. Neurochem Res 46, 1627–1640 (2021). https://doi.org/10.1007/s11064-021-03273-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03273-6