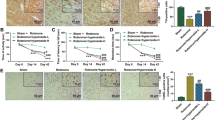
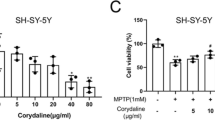
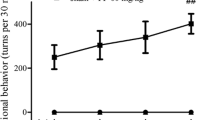
Abstract
Recent researches have shown that autophagy is associated with the pathogenesis of neurodegenerative disorders, but there is no paper to investigate the effects of autophagy modulation on Parkinson’s disease depression (PDD). In addition, glycyrrhizic acid (GA), the major bioactive ingredient of Radix glycyrrhizae, can induce autophagy and ease rotenone-induced Parkinson’s disease (PD). However, there is also no paper to study the action and molecular mechanisms of GA on PDD. In this research, we built the injury model of SH-SY5Y cells through 6-hydroxydopamine (6-OHDA) and corticosterone (CORT). Then, our results showed that GA markedly increased the viability and decreased the apoptosis in SH-SY5Y cells after pre-treating with 6-OHDA and CORT. Moreover, GA notably decreased the expressions of α-Syn and p-S1292-LRRK2 proteins, and significantly increased the levels of CREB and BDNF proteins. Previous papers have suggested that CORT contributed to dopaminergic neurodegeneration via the glucocorticoid (GC)/glucocorticoid receptor (GR) interaction, and our results showed that GA reduced GC level and hypothalamic–pituitary–adrenal (HPA) activity in SH-SY5Y cells by regulating GR signaling pathway. Furthermore, mechanism investigations also showed that GA had the ability to up-regulate the conversion of LC3B II/I and the expression of Beclin-1, and induce autophagy in SH-SY5Y cells, which were reversed by the autophagy inhibitor 3-methyladenine (3-MA). Collectively, these findings proved that GA exerted efficient activity against neurotoxicity in SH-SY5Y cells induced by 6-OHDA and CORT via activation of autophagy, which should be developed as an efficient candidate for treating PDD in the future.








Similar content being viewed by others
References
Marsh L (2013) Depression and Parkinson’s disease: current knowledge. Curr Neurol Neurosci Rep 13(12):409. https://doi.org/10.1007/s11910-013-0409-5
Rihmer Z, Gonda X, Dome P (2014) Depression in Parkinson’s disease. Ideggyogyaszati szemle 67(7–8):229–236
Timmer MHM, van Beek M, Bloem BR, Esselink RAJ (2017) What a neurologist should know about depression in Parkinson’s disease. Pract Neurol 17(5):359–368. https://doi.org/10.1136/practneurol-2017-001650
Ishihara L, Brayne C (2006) A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol Scand 113(4):211–220. https://doi.org/10.1111/j.1600-0404.2006.00579.x
Aarsland D, Pahlhagen S, Ballard CG, Ehrt U, Svenningsson P (2011) Depression in Parkinson disease–epidemiology, mechanisms and management. Nat Rev Neurol 8(1):35–47. https://doi.org/10.1038/nrneurol.2011.189
Dobkin RD, Menza M, Bienfait KL, Gara M, Marin H, Mark MH, Dicke A, Friedman J (2011) Depression in Parkinson’s disease: symptom improvement and residual symptoms after acute pharmacologic management. Am J Geriatr Psychiatry 19(3):222–229. https://doi.org/10.1097/JGP.0b013e3181e448f7
Dalle E, Mabandla MV (2018) Early life stress, depression and Parkinson’s disease: a new approach. Mol Brain 11(1):18. https://doi.org/10.1186/s13041-018-0356-9
Dobkin RD, Menza M, Bienfait KL, Gara M, Marin H, Mark MH, Dicke A, Troster A (2010) The impact of antidepressant treatment on cognitive functioning in depressed patients with Parkinson’s disease. J Neuropsychiatry Clin Neurosci 22(2):188–195. https://doi.org/10.1176/appi.neuropsych.22.2.188
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. https://doi.org/10.1038/nature04723
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Ann Rev Neurosci 28:57–87. https://doi.org/10.1146/annurev.neuro.28.061604.135718
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302(5646):819–822. https://doi.org/10.1126/science.1087753
Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, Ding JQ, Chen SD (2013) Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J Neuroimmune Pharmacol 8(1):356–369. https://doi.org/10.1007/s11481-012-9431-7
Sandal M, Valle F, Tessari I, Mammi S, Bergantino E, Musiani F, Brucale M, Bubacco L, Samori B (2008) Conformational equilibria in monomeric alpha-synuclein at the single-molecule level. PLoS Biol 6(1):e6. https://doi.org/10.1371/journal.pbio.0060006
Wirawan E, Vanden Berghe T, Lippens S, Agostinis P, Vandenabeele P (2012) Autophagy: for better or for worse. Cell Res 22(1):43–61. https://doi.org/10.1038/cr.2011.152
Kunugi H, Hori H, Adachi N, Numakawa T (2010) Interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression. Psychiatry Clin Neurosci 64(5):447–459. https://doi.org/10.1111/j.1440-1819.2010.02135.x
Dwivedi Y (2013) Involvement of brain-derived neurotrophic factor in late-life depression. Am J Geriatr Psychiatry 21(5):433–449. https://doi.org/10.1016/j.jagp.2012.10.026
Bai Y, Song L, Dai G, Xu M, Zhu L, Zhang W, Jing W, Ju W (2018) Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection. Steroids. https://doi.org/10.1016/j.steroids.2018.03.005
Johnson SA, Fournier NM, Kalynchuk LE (2006) Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res 168(2):280–288. https://doi.org/10.1016/j.bbr.2005.11.019
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581(1–2):113–120. https://doi.org/10.1016/j.ejphar.2007.12.005
Ojha S, Javed H, Azimullah S, Abul Khair SB, Haque ME (2016) Glycyrrhizic acid attenuates neuroinflammation and oxidative stress in rotenone model of Parkinson’s disease. Neurotox Res 29(2):275–287. https://doi.org/10.1007/s12640-015-9579-z
Xicoy H, Wieringa B, Martens GJ (2017) The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener 12(1):10. https://doi.org/10.1186/s13024-017-0149-0
Hao Y, Shabanpoor A, Metz GA (2017) Stress and corticosterone alter synaptic plasticity in a rat model of Parkinson’s disease. Neurosci Lett 651:79–87. https://doi.org/10.1016/j.neulet.2017.04.063
Qiu G, Spangler EL, Wan R, Miller M, Mattson MP, So KF, de Cabo R, Zou S, Ingram DK (2012) Neuroprotection provided by dietary restriction in rats is further enhanced by reducing glucocortocoids. Neurobiol Aging 33(10):2398–2410. https://doi.org/10.1016/j.neurobiolaging.2011.11.025
Liu ZR, Sun LZ, Jia TH, Jia DF (2017) beta-Aescin shows potent antiproliferative activity in osteosarcoma cells by inducing autophagy, ROS generation and mitochondrial membrane potential loss. J BUON 22(6):1582–1586
Wang S, Ge W, Harns C, Meng X, Zhang Y, Ren J (2018) Ablation of toll-like receptor 4 attenuates aging-induced myocardial remodeling and contractile dysfunction through NCoRI-HDAC1-mediated regulation of autophagy. J Mol Cell Cardiol 119:40–50. https://doi.org/10.1016/j.yjmcc.2018.04.009
Schneider L, Giordano S, Zelickson BR, M SJ, Ouyang GAB, Fineberg X, Darley-Usmar N, Zhang VM J (2011) Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic Biol Med 51(11):2007–2017. https://doi.org/10.1016/j.freeradbiomed.2011.08.030
Xie HR, Hu LS, Li GY (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J 123(8):1086–1092
Fan Y, Howden AJM, Sarhan AR, Lis P, Ito G, Martinez TN, Brockmann K, Gasser T, Alessi DR, Sammler EM (2018) Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem J 475(1):23–44. https://doi.org/10.1042/bcj20170803
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44(4):601–607. https://doi.org/10.1016/j.neuron.2004.11.005
Bardien S, Lesage S, Brice A, Carr J (2011) Genetic characteristics of leucine-rich repeat kinase 2 (LRRK2) associated Parkinson’s disease. Parkinsonism Relat Disord 17(7):501–508. https://doi.org/10.1016/j.parkreldis.2010.11.008
Lavalley NJ, Slone SR, Ding H, West AB, Yacoubian TA (2016) 14-3-3 Proteins regulate mutant LRRK2 kinase activity and neurite shortening. Hum Mol Genet 25(1):109–122. https://doi.org/10.1093/hmg/ddv453
Correia Guedes L, Ferreira JJ, Rosa MM, Coelho M, Bonifati V, Sampaio C (2010) Worldwide frequency of G2019S LRRK2 mutation in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord 16(4):237–242. https://doi.org/10.1016/j.parkreldis.2009.11.004
Qi G, Mi Y, Wang Y, Li R, Huang S, Li X, Liu X (2017) Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct 8(12):4421–4432. https://doi.org/10.1039/c7fo00991g
Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front Mol Neurosci 3:1. https://doi.org/10.3389/neuro.02.001.2010
Ramamoorthy S, Cidlowski JA (2013) Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr Dev 24:41–56. https://doi.org/10.1159/000342502
West DC, Kocherginsky M, Tonsing-Carter EY, Dolcen DN, Hosfield DJ, Lastra RR, Sinnwell JP, Thompson KJ, Bowie KR, Harkless RV, Skor MN, Pierce CF, Styke SC, Kim CR, de Wet L, Greene GL (2018) Discovery of a glucocorticoid receptor (GR) activity signature using selective GR antagonism in ER-negative breast cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.ccr-17-2793
Fries GR, Gassen NC, Rein T (2017) The FKBP51 glucocorticoid receptor co-chaperone: regulation, function, and implications in health and disease. Int J Mol Sci. https://doi.org/10.3390/ijms18122614
Funding
This work was supported by National Natural Science foundation of China (No. 81573922), Hubei province technical innovation project (No. 2017ACA176), Natural Science Foundation of Guangdong Province (No. 2017ZC0135) and Elite Youth Education Program of Guangzhou University of Chinese Medicine (No. QNYC20140104).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, G., Li, J., Cai, Y. et al. Glycyrrhizic Acid Alleviates 6-Hydroxydopamine and Corticosterone-Induced Neurotoxicity in SH-SY5Y Cells Through Modulating Autophagy. Neurochem Res 43, 1914–1926 (2018). https://doi.org/10.1007/s11064-018-2609-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2609-5