Abstract
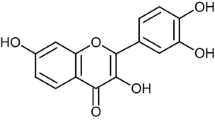
Gallic acid is one of the most important polyphenolic compounds, which is considered an excellent free radical scavenger. 6-Hydroxydopamine (6-OHDA) is a neurotoxin, which has been implicated in mainly Parkinson’s disease (PD). In this study, we investigated the molecular mechanism of the neuroprotective effects of gallic acid on 6-OHDA induced apoptosis in human dopaminergic cells, SH-SY5Y. Our results showed that 6-OHDA induced cytotoxicity in SH-SY5Y cells was suppressed by pre-treatment with gallic acid. The percentage of live cells (90%) was high in the pre-treatment of gallic acid when compared with 6-OHDA alone treated cell line. Moreover, gallic acid was very effective in attenuating the disruption of mitochondrial membrane potential, elevated levels of intracellular ROS and apoptotic cell death induced by 6-OHDA. Gallic acid also lowered the ratio of the pro-apoptotic Bax protein and the anti-apoptotic Bcl-2 protein in SH-SY5Y cells. 6-OHDA exposure was up-regulated caspase-3 and Keap-1 and, down-regulated Nrf2, BDNF and p-CREB, which were sufficiently reverted by gallic acid pre-treatment. These findings indicate that gallic acid is able to protect the neuronal cells against 6-OHDA induced injury and proved that gallic acid might potentially serve as an agent for prevention of several human neurodegenerative diseases caused by oxidative stress and apoptosis.








Similar content being viewed by others
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- LDH:
-
Lactate dehydrogenase
- GA:
-
Gallic acid
- DCDFA:
-
2′,7′-Dichlorofluorescein diacetate
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ΔΨm:
-
Mitochondrial membrane potential
References
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222
Mullin S, Schapira AHV (2015) Pathogenic mechanisms of neurogeneration in Parkinson disease. Neurol Clin 33:1–17
Hu Y, Tong Y (2010) A Trojan horse for Parkinson’s disease. Sci Signal 3:p13–pe13
Chong SJF, Low ICC, Pervaiz S (2014) Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 19:39–48
Navya K, Kumar GP, Anilakumar KR (2017) Ameliorating effect of Curculigo orchoides on chromium(VI) induced oxidative stress via, modulation of cytokines, transcription factors and apoptotic genes. J Appl Biomed 15:299–306
Jiang XW, Bai JP, Zhang Q, Hu XL, Tian X, Zhu J, Liu J, Meng WH, Zhao QC (2017) Caffeoylquinic acid derivatives protect SH-SY5Y neuroblastoma cells from hydrogen peroxide-induced injury through modulating oxidative status. Cell Mol Neurobiol 37:499–509
Kumar GP, Anilakumar KR, Naveen S (2015) Phytochemicals having neuroprotective properties from dietary sources and medicinal herbs. Pharmacogn J 7:1–17
Mazo NA, Echeverria V, Cabezas R, Avila-Rodriguez M, Aliev G, Leszek J, Barreto GE (2017) Medicinal plants as protective strategies against Parkinson’s disease. Curr Pharm Des 23:4180–4188
Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC (2013) Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine 80:179–274
Umadevi S, Gopi V, Simna S, Parthasarathy A, Yousuf SMJ, Elangova V (2012) Studies on the cardio protective role of gallic acid against age-induced cell proliferation and oxidative stress in H9C2 (2–1) cells. Cardiovasc Toxicol 12:304–311
Rasool MK, Sabina EP, Ramya SR, Preety P, Patel S, Mandal N, Mishra PP, Samuel J (2010) Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J Pharm Pharmcol 62:638–643
Dhingra D, Chhillar R, Gupta A (2012) Antianxiety-like activity of gallic acid in unstressed and stressed mice: possible involvement of nitriergic system. Neurochem Res 37:487–494
Tieu K (2011) A guide to neurotoxic animal models of Parkinson’s disease. Csh Perspect Med 1:a009316
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Ramya EM, Kumar GP, Anand T, Anilakumar KR (2017) Modulatory effects of Terminalia arjuna against domoic acid induced toxicity in Caco-2 cell line. Cytotechnology 69:725–739
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Liu Y, Zeng X, Hui Y, Zhu C, Wu J, Taylor DH, Ji J, Fan W, Huang Z, Hu J (2015) Activation of α7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson’s disease. Neuropharmacology 91:87–96
Sunkaria A, Yadav A, Sharma SK, Sandhir R (2017) Molecular mechanisms involved in the neuroprotective action of phytochemicals. Neuroprot Eff Phytochem Neurol Disord 515–559
Sekhar YC, Kumar GP, Anilakumar KR (2017) Terminalia arjuna bark extract attenuates picrotoxin-induced behavioral changes by activation of serotonergic, dopaminergic, GAB Aergic and antioxidant systems. Chin J Nat Medicines 15:584–596
Workman DG, Tsatsanis A, Lewis FW, Boyle JP, Mousadoust M, Hettiarachchi NT, Hunter M, Peers CS, Tétard D, Duce JA (2015) Protection from neurodegeneration in the 6-hydroxydopamine (6-OHDA) model of Parkinson’s with novel 1-hydroxypyridin-2-one metal chelators. Metallomics 7:867–876
Izumi Y, Sawada H, Sakka N, Yamamoto N, Kume T, Katsuki H, Shimohama S, Akaike A (2005) p-quinone mediates 6-hydroxydopamine-induced dopaminergic neuronal death and ferrous iron accelerates the conversion of p-quinone into melanin extracellularly. J Neurosci Res 79:849–860
Xie L, Hu LF, Teo XQ, Tiong CX, Tazzari V, Sparatore A, Del Soldato P, Dawe GS, Bian JS (2013) Therapeutic effect of hydrogen sulfide-releasing L-Dopa derivative ACS84 on 6-OHDA-induced Parkinson’s disease rat model. PLoS ONE 8:e60200
Naoi M, Inaba-Hasegawa K, Shamoto-Nagai M, Maruyama W (2017) Neurotrophic function of phytochemicals for neuroprotection in aging and neurodegenerative disorders: modulation of intracellular signalling and gene expression. J Neural Transm 124:1515–1527
Nie G, Cao Y, Zhao B (2002) Protective effects of green tea polyphenols and their major component, (–)-epigallocatechin-3-gallate (EGCG), on 6-hydroxydopamine-induced apoptosis in PC12 cells. Redox Rep 7:171–177
Cohen JJ, Duke RC, Fadok VA, Sellins KS (1992) Apoptosis and programmed cell death in immunity. Annu Rev Immunol 10:267–293
Tortosa A, Lopez E, Ferrer I (1997) Bcl-2 and Bax proteins in Lewy bodies from patients with Parkinson’s disease and diffuse Lewy body disease. Neurosci Lett 238:78–80
Vander Heiden MG, Thompson CB (1999) Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol 1:E209
Hwang DS, Kim HG, Kwon HJ, Cho JH, Lee CH, Lee JM, Jang JB, Kim YS, Lee KS, Oh MS (2011) Dangguijakyak-san, a medicinal herbal formula, protects dopaminergic neurons from 6-hydroxydopamine-induced neurotoxicity. J Ethnopharmacol 133:934–939
Zhang Z, Hou L, Li X, Ju C, Zhang J, Li X, Wang X, Liu C, Lv Y, Wang Y (2016) Neuroprotection of inositol hexaphosphate and changes of mitochondrion mediated apoptotic pathway and α-synuclein aggregation in 6-OHDA induced parkinson׳ s disease cell model. Brain Res 1633:87–95
Wu CR, Tsai CW, Chang SW, Lin CY, Huang LC (2015) Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: involvement of antioxidative enzymes induction. Chem-Biol Interact 225:40–46
Miller DM, Singh IN, Wang JA, Hall ED (2013) Administration of the Nrf2–ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radical Bio Med 57:1–9
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radical Bio Med 88:179–188
Wang H, Wang Y, Zhao L, Cui Q, Wang Y, Du G (2016) Pinocembrin attenuates MPP+-induced neurotoxicity by the induction of heme oxygenase-1 through ERK1/2 pathway. Neurosci Lett 612:104–109
Wang H, Liu X, Long M, Huang Y, Zhang L, Zhang R, Zheng Y, Liao X, Wang Y, Liao Q, Li W (2016) NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci Transl Med 8:334ra51
Qi G, Mi Y, Wang Y, Li R, Huang S, Li X, Liu X (2017) Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct 8:4421–4432
Chao J, Lau WK, Huie MJ, Ho YS, Yu MS, Lai CS, Wang M, Yuen WH, Lam WH, Chan TH, Chang RC (2010) A pro-drug of the green tea polyphenol (–)-epigallocatechin-3-gallate (EGCG) prevents differentiated SH-SY5Y cells from toxicity induced by 6-hydroxydopamine. Neurosci Lett 469:360–364
Acknowledgements
We would like to thank Director, DFRL (DRDO) for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interests.
Rights and permissions
About this article
Cite this article
Chandrasekhar, Y., Phani Kumar, G., Ramya, E.M. et al. Gallic Acid Protects 6-OHDA Induced Neurotoxicity by Attenuating Oxidative Stress in Human Dopaminergic Cell Line. Neurochem Res 43, 1150–1160 (2018). https://doi.org/10.1007/s11064-018-2530-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2530-y