Abstract
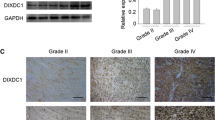
Dual-specificity tyrosine-regulated kinase 2 (DYRK2), a protein kinase that phosphorylates its substrates on serine/threonine, is expressed in numerous human tumors, but little is known about its role in the pathophysiology of glioma. In this study, we made an effort to explore the expression and function in human glioma. Western blot and immunohistochemistry analysis were performed to investigate the expression of DYRK2 protein in glioma tissues in 84 patients. Wound healing and transwell assay were carried out to determine the cell migration ability. We showed that the level of DYRK2 was significantly decreased in high-grade glioma tissues compared with low-grade tissues. In addition, the expression level of DYRK2 was positively correlated with glioma pathological grade and E-cadherin expression. Kaplane–Meier analysis revealed that low expression of DYRK2 was related to poor prognosis of glioma patients. Furthermore, wound healing and transwell assay revealed that DYRK2 could suppress cell migration and affect the expression levels of E-cadherin and vimentin through PI3K/AKT/GSK3β signaling pathway. Taken together, our results implied that DYRK2 could serve as a promising prognostic biomarker as well as a potential therapeutical target of glioma.






Similar content being viewed by others
References
Jovcevska I, Kocevar N, Komel R (2013) Glioma and glioblastoma—how much do we (not) know? Mol Clin Oncol 1:935–941. doi:10.3892/mco.2013.172
Parker NR, Khong P, Parkinson JF, Howell VM, Wheeler HR (2015) Molecular heterogeneity in glioblastoma: potential clinical implications. Front Oncol 5:55. doi:10.3389/fonc.2015.00055
Mrugala MM (2013) Advances and challenges in the treatment of glioblastoma: a clinician’s perspective. Discov Med 15:221–230
Miller CT, Aggarwal S, Lin TK, Dagenais SL, Contreras JI, Orringer MB, Glover TW, Beer DG, Lin L (2003) Amplification and overexpression of the dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 2 (DYRK2) gene in esophageal and lung adenocarcinomas. Cancer Res 63:4136–4143
Enomoto Y, Yamashita S, Yoshinaga Y, Fukami Y, Miyahara S, Nabeshima K, Iwasaki A (2014) Downregulation of DYRK2 can be a predictor of recurrence in early stage breast cancer. Tumour Biol 35:11021–11025. doi:10.1007/s13277-014-2413-z
Yamashita S, Chujo M, Moroga T, Anami K, Tokuishi K, Miyawaki M, Kawano Y, Takeno S, Yamamoto S, Kawahara K (2009) DYRK2 expression may be a predictive marker for chemotherapy in non-small cell lung cancer. Anticancer Res 29:2753–2757
Yamaguchi N, Mimoto R, Yanaihara N, Imawari Y, Hirooka S, Okamoto A, Yoshida K (2015) DYRK2 regulates epithelial-mesenchymal-transition and chemosensitivity through Snail degradation in ovarian serous adenocarcinoma. Tumour Biol 36:5913–5923. doi:10.1007/s13277-015-3264-y
Aranda S, Laguna A and de la Luna S (2011) DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J 25:449–462. doi:10.1096/fj.10-165837
Mimoto R, Taira N, Takahashi H, Yamaguchi T, Okabe M, Uchida K, Miki Y, Yoshida K (2013) DYRK2 controls the epithelial-mesenchymal transition in breast cancer by degrading Snail. Cancer Lett 339:214–225. doi:10.1016/j.canlet.2013.06.005
Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A (2006) A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441:646–650. doi:10.1038/nature04631
Varjosalo M, Bjorklund M, Cheng F, Syvanen H, Kivioja T, Kilpinen S, Sun Z, Kallioniemi O, Stunnenberg HG, He WW, Ojala P, Taipale J (2008) Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell 133:537–548. doi:10.1016/j.cell.2008.02.047
Perez M, Garcia-Limones C, Zapico I, Marina A, Schmitz ML, Munoz E, Calzado MA (2012) Mutual regulation between SIAH2 and DYRK2 controls hypoxic and genotoxic signaling pathways. J Mol Cell Biol 4:316–330. doi:10.1093/jmcb/mjs047
Taira N, Nihira K, Yamaguchi T, Miki Y, Yoshida K (2007) DYRK2 is targeted to the nucleus and controls p53 via Ser46 phosphorylation in the apoptotic response to DNA damage. Mol Cell 25:725–738. doi:10.1016/j.molcel.2007.02.007
Weiss CS, Ochs MM, Hagenmueller M, Streit MR, Malekar P, Riffel JH, Buss SJ, Weiss KH, Sadoshima J, Katus HA, Hardt SE (2013) DYRK2 negatively regulates cardiomyocyte growth by mediating repressor function of GSK-3beta on eIF2Bepsilon. PLoS ONE 8:e70848. doi:10.1371/journal.pone.0070848
Zhong Z, Hu Z, Jiang Y, Sun R, Chen X, Chu H, Zeng M, Sun C (2016) Interleukin-11 promotes epithelial-mesenchymal transition in anaplastic thyroid carcinoma cells through PI3K/Akt/GSK3beta signaling pathway activation. Oncotarget. doi:10.18632/oncotarget.10831
Dai J, Qian C, Su M, Chen M, Chen J (2016) Gastrokine-2 suppresses epithelial mesenchymal transition through PI3K/AKT/GSK3beta signaling in gastric cancer. Tumour Biol. doi:10.1007/s13277-016-5107-x
Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C (2011) Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol 13:1339–1348. doi:10.1093/neuonc/nor133
Houdek Z, Cendelin J, Kulda V, Babuska V, Cedikova M, Kralickova M, Pachernik J, Stefano GB, Vozeh F (2012) Intracerebellar application of P19-derived neuroprogenitor and naive stem cells to Lurcher mutant and wild type B6CBA mice. Med Sci Monit 18:BR174–B180
Jansen M, Yip S, Louis DN (2010) Molecular pathology in adult gliomas: diagnostic, prognostic, and predictive markers. Lancet Neurol 9:717–726. doi:10.1016/S1474-4422(10)70105-8
Rock K, McArdle O, Forde P, Dunne M, Fitzpatrick D, O’Neill B, Faul C (2012) A clinical review of treatment outcomes in glioblastoma multiforme—the validation in a non-trial population of the results of a randomised Phase III clinical trial: has a more radical approach improved survival? Br J Radiol 85:e729–e733. doi:10.1259/bjr/83796755
Wang Y, Wu Y, Miao X, Zhu X, Miao X, He Y, Zhong F, Ding L, Liu J, Tang J, Huang Y, Xu X, He S (2015) Silencing of DYRK2 increases cell proliferation but reverses CAM-DR in non-Hodgkin’s lymphoma. Int J Biol Macromol 81:809–817. doi:10.1016/j.ijbiomac.2015.08.067
Nomura S, Suzuki Y, Takahashi R, Terasaki M, Kimata R, Terasaki Y, Hamasaki T, Kimura G, Shimizu A, Kondo Y (2015) Dual-specificity tyrosine phosphorylation-regulated kinase 2 (DYRK2) as a novel marker in T1 high-grade and T2 bladder cancer patients receiving neoadjuvant chemotherapy. BMC Urol 15:53. doi:10.1186/s12894-015-0040-7
van Roy F, Berx G (2008) The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 65:3756–3788. doi:10.1007/s00018-008-8281-1
Wijnhoven BP, Dinjens WN, Pignatelli M (2000) E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 87:992–1005. doi:10.1046/j.1365-2168.2000.01513.x
Yang L, Liu M, Deng C, Gu Z, Gao Y (2012) Expression of transforming growth factor-beta1 (TGF-beta1) and E-cadherin in glioma. Tumour Biol 33:1477–1484. doi:10.1007/s13277-012-0398-z
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, Fan J, Dai Z, Zhou J (2015) CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3beta/snail signaling. Cancer Lett 358:124–135. doi:10.1016/j.canlet.2014.11.044
Liang Y, Jing Z, Deng H, Li Z, Zhuang Z, Wang S, Wang Y (2015) Soluble epoxide hydrolase inhibition ameliorates proteinuria-induced epithelial-mesenchymal transition by regulating the PI3K-Akt-GSK-3beta signaling pathway. Biochem Biophys Res Commun 463:70–75. doi:10.1016/j.bbrc.2015.05.020
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81502169, 81572491, 81373223, and 81571616); Technology Innovation Programme of Jiangsu Province (KYZZ15_0351), Technology Innovation Programme of Nantong University (YKC15068) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, Y., Zhang, L., Wang, D. et al. Regulation of Glioma Cells Migration by DYRK2. Neurochem Res 42, 3093–3102 (2017). https://doi.org/10.1007/s11064-017-2345-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2345-2