Abstract
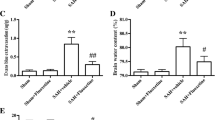
As is known to all, neuroinflammation plays a vital role in early brain injury pathogenesis following subarachnoid hemorrhage (SAH). It has been shown that rutin have a property of inhibiting inflammation in many kinds of animal models. However, the effect of rutin on neuroinflammation after SAH remains uninvestigated. In this study, we investigated the potential effects of rutin on neuroinflammation and the underlying mechanism in an experimental rat model of SAH performed by endovascular perforation. Adult male SD rats were randomly divided into three groups, including sham group, SAH + vehicle group and SAH + rutin group (50 mg/kg) intraperitoneally (i.p.) administered at 30 min after SAH. After sacrificed at 24 h after SAH, all rats were examined by following tests, including neurologic scores, blood–brain barrier permeability, brain water content and neuronal cell death in cerebral cortex. The level of inflammation in brain was estimated by means of multiple molecules, including RAGE, NF-κB, and inflammation cytokines. Our results indicated that rutin could significantly downregulate the increased level of REGE, NF-κB and inflammatory cytokines in protein level. In addition, rutin could also ameliorate a series of secondary brain injuries such as brain edema, destruction of blood–brain barrier, neurological deficits and neuronal death. This study indicated that rutin administration had a neuroprotective effect in an experimental rat model of SAH, possibly through inhibiting RAGE–NF-κB mediated inflammation signaling pathway.




Similar content being viewed by others
References
van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369(9558):306–318
Velat GJ, Kimball MM, Mocco JD, Hoh BL (2011) Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg 76(5):446–454
Cahill J, Zhang JH (2009) Subarachnoid hemorrhage: is it time for a new direction? Stroke 40(3 Suppl):S86–S87
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97(1):14–37
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26(11):1341–1353
Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S et al (2009) Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke 40(7):2519–2525
Yan SF, Yan SD, Ramasamy R, Schmidt AM (2009) Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med 41(6):408–422
Ramasamy R, Yan SF, Schmidt AM (2009) RAGE: therapeutic target and biomarker of the inflammatory response–the evidence mounts. J Leukoc Biol 86(3):505–512
Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A et al (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382(6593):685–691
Bianchi R, Giambanco I, Donato R (2010) S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging 31(4):665–677
Uivarosi V, Barbuceanu SF, Aldea V, Arama CC, Badea M, Olar R et al (2010) Synthesis, spectral and thermal studies of new rutin vanadyl complexes. Molecules 15(3):1578–1589
Isai M, Sakthivel M, Ramesh E, Thomas PA, Geraldine P (2009) Prevention of selenite-induced cataractogenesis by rutin in Wistar rats. Mol Vis 15:2570–2577
Sharma S, Ali A, Ali J, Sahni JK, Baboota S (2013) Rutin: therapeutic potential and recent advances in drug delivery. Expert Opin Investig Drugs 22(8):1063–1079
Khan MM, Ahmad A, Ishrat T, Khuwaja G, Srivastawa P, Khan MB et al (2009) Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res 1292:123–135
Jeong JJ, Ha YM, Jin YC, Lee EJ, Kim JS, Kim HJ et al (2009) Rutin from Lonicera japonica inhibits myocardial ischemia/reperfusion-induced apoptosis in vivo and protects H9c2 cells against hydrogen peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro. Food Chem Toxicol 47(7):1569–1576
Korkmaz A, Kolankaya D (2010) Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res 164(2):309–315
Jang JW, Lee JK, Hur H, Kim TW, Joo SP, Piao MS (2014) Rutin improves functional outcome via reducing the elevated matrix metalloproteinase-9 level in a photothrombotic focal ischemic model of rats. J Neurol Sci 339(1–2):75–80
Rodrigues AM, dos Santos Marcilio F, Frazao Muzitano M, Giraldi-Guimaraes A (2013) Therapeutic potential of treatment with the flavonoid rutin after cortical focal ischemia in rats. Brain Res 1503:53–61
Nafees S, Rashid S, Ali N, Hasan SK, Sultana S (2015) Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFkappaB/MAPK pathway. Chem Biol Interact 231:98–107
Yamaguchi M, Zhou C, Nanda A, Zhang JH (2004) Ras protein contributes to cerebral vasospasm in a canine double-hemorrhage model. Stroke 35(7):1750–1755
Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M (1988) Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab 8(2):282–284
You WC, Li W, Zhuang Z, Tang Y, Lu HC, Ji XJ et al (2012) Biphasic activation of nuclear factor-kappa B in experimental models of subarachnoid hemorrhage in vivo and in vitro. Mediators Inflamm 2012:786242
Zhuang Z, Zhou ML, You WC, Zhu L, Ma CY, Sun XJ et al (2012) Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci 13:47
Ostrowski RP, Colohan AR, Zhang JH (2005) Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab 25(5):554–571
Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25(7):1342–1347
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28(4):399–414
Sehba FA, Pluta RM, Zhang JH (2011) Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol 43(1):27–40
Church LD, Churchman SM, Hawkins PN, McDermott MF (2006) Hereditary auto-inflammatory disorders and biologics. Springer Semin Immunopathol 27(4):494–508
Lockyer JM, Colladay JS, Alperin-Lea WL, Hammond T, Buda AJ (1998) Inhibition of nuclear factor-kappaB-mediated adhesion molecule expression in human endothelial cells. Circ Res 82(3):314–320
Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S (2004) Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 24(11):2137–2142
Takeda R, Suzuki E, Satonaka H, Oba S, Nishimatsu H, Omata M et al (2005) Blockade of endogenous cytokines mitigates neointimal formation in obese Zucker rats. Circulation 111(11):1398–1406
Arjumand W, Seth A, Sultana S (2011) Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFkappaB, TNF-alpha and caspase-3 expression in wistar rats. Food Chem Toxicol 49(9):2013–2021
Yeh CH, Yang JJ, Yang ML, Li YC, Kuan YH (2014) Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-kappaB pathway. Free Radic Biol Med 69:249–257
Annapurna A, Ansari MA, Manjunath PM (2013) Partial role of multiple pathways in infarct size limiting effect of quercetin and rutin against cerebral ischemia-reperfusion injury in rats. Eur Rev Med Pharmacol Sci 17(4):491–500
Qu J, Zhou Q, Du Y, Zhang W, Bai M, Zhang Z et al (2014) Rutin protects against cognitive deficits and brain damage in rats with chronic cerebral hypoperfusion. Br J Pharmacol 171(15):3702–3715
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5(4):331–342
Hudson BI, Harja E, Moser B, Schmidt AM (2005) Soluble levels of receptor for advanced glycation endproducts (sRAGE) and coronary artery disease: the next C-reactive protein? Arterioscler Thromb Vasc Biol 25(5):879–882
Kalea AZ, Schmidt AM, Hudson BI (2009) RAGE: a novel biological and genetic marker for vascular disease. Clin Sci (Lond). 116(8):621–637
Li J, Schmidt AM (1997) Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem 272(26):16498–16506
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any conflict of interest.
Additional information
Guangzhi Hao and Yushu Dong have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hao, G., Dong, Y., Huo, R. et al. Rutin Inhibits Neuroinflammation and Provides Neuroprotection in an Experimental Rat Model of Subarachnoid Hemorrhage, Possibly Through Suppressing the RAGE–NF-κB Inflammatory Signaling Pathway. Neurochem Res 41, 1496–1504 (2016). https://doi.org/10.1007/s11064-016-1863-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1863-7