Abstract
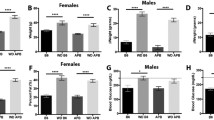
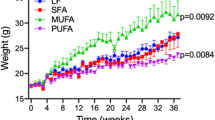
It has been suggested that advanced glycation end (AGE) products, via cognate receptor activation, are implicated in several diseases, including Alzheimer’s disease. The NMDA receptor–nitric oxide pathway appears to be influenced by AGE products and involved in the pathogenesis of this type of dementia. In this study, C57BL/6J (WT) and transgenic (Tg2576) mice expressing human mutant amyloid precursor protein were kept on prolonged (8 months) diets containing regular or high amounts of AGE products. After the decapitation of 11-months old mice, brain tissue analyses were performed [expressions of the NR1, NR2A and NR2B subunits of NMDA receptors, activities of neuronal, endothelial and inducible nitric oxide synthase (nNOS, eNOS and iNOS)]. Moreover, levels of malondialdehyde and of human amyloid β 1–42 were estimated. We found increased activity of nNOS in WT mice maintained on a high compared to regular AGE diet; however, no similar differences were found in Tg2576 mice. In addition, we observed an increase in NR1 expression in Tg2576 compared to WT mice, both kept on a diet high in AGE products. Correlation analyses performed on mice kept on the regular AGE diet supported close links between particular subunits (NR2A–NR2B, in WT as well as in Tg2576 mice), between subunits and synthase (NR2A/NR2B–nNOS, only in WT mice) or between particular synthases (nNOS–iNOS, only in WT). Correlation analysis also revealed differences between WT mice kept on both diets (changed correlations between NR2A/NR2B–nNOS, between nNOS–eNOS and between eNOS–iNOS). Malondialdehyde levels were increased in both Tg2576 groups when compared to the corresponding WT mice, but no effects of the diets were observed. Analogously, no significant effects of diets were found in the levels of soluble or insoluble amyloid β 1–42 in Tg2576 mice. Our results demonstrate that prolonged ingestion of AGE products can influence the NMDA receptor–nitric oxide pathway in the brain and that only WT mice, not Tg2576 mice, are able to maintain homeostasis among subunits and synthases or among particular synthases. The prolonged application of AGE products enhanced differences between 11-months old Tg2576 and WT mice regarding this pathway. Observed differences in the pathway between WT mice kept on regular or high AGE diets suggest that the prolonged application of a diet low in AGE products could have beneficial effects in older or diabetic people and perhaps also in people with Alzheimer’s disease.


Similar content being viewed by others
References
Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382:685–691
Shuvaev VV, Laffont I, Serot JM, Fujii J, Taniguchi N, Siest G (2001) Increased protein glycation in cerebrospinal fluid of Alzheimer’s disease. Neurobiol Aging 22:397–402
Yan SD, Bierhaus A, Nawroth PP, Stern DM (2009) RAGE and Alzheimer’s disease: a progression factor for amyloid-beta-induced cellular perturbation? J Alzheimers Dis 16:833–843
Yang Y, Song W (2013) Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 250:140–150
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutlege R, Lin B, Amoscato AA, Zeh HJ, Lotze MT (2009) RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7:17. doi:10.1186/1479-5876-7-17
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA 91:4766–4770
Kroner Z (2009) The relationship between Alzheimer’s disease and diabetes: type 3 diabetes? Altern Med Rev 14:373–379
Pappolla MA, Alzofon J, McMahon J, Theodoropoulos TJ (1990) Ultrastructural evidence that insoluble microtubules are components of the neurofibrillary tangle. Eur Arch Psychiatry Neurol Sci 239:314–319
Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G (1994) Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA 91:5710–5714
Kristofikova Z, Kozmikova I, Hovorkova P, Ricny J, Zach P, Majer E, Klaschka J, Ripova D (2008) Lateralization of hippocampal nitric oxide mediator system in people with Alzheimer disease, multi-infarct dementia and schizophrenia. Neurochem Int 53:118–125
Kristofikova Z, Vrajova M, Sirova J, Vales K, Petrasek T, Schönig K, Tews B, Schwab M, Bartsch D, Stuchlik A, Ripova D (2013) N-methyl-d-aspartate receptor–nitric oxide synthase pathway in the cortex of Nogo-A-deficient rats in relation to brain laterality and schizophrenia. Front Behav Neurosci 7:90. doi:10.3389/fnbeh.2013.00090
Kamida T, Takeda Y, Fujiki M, Abe T, Abe E, Kobayashi H (2007) Nitric oxide synthase and NMDA receptor expressions in cavernoma tissues with epileptogenesis. Acta Neurol Scand 116:368–373
Riederer P, Hoyer S (2006) From benefit to damage. Glutamate and advanced glycation end products in Alzheimer brain. J Neural Transm 113:1671–1677
Ryan CM, Geckle M (2000) Why is learning and memory dysfunction in Type 2 diabetes limited to older adults? Diabetes Metab Res Rev 16:308–315
Soares E, Prediger RD, Nunes S, Castro AA, Viana SD, Lemos C, De Souza CM, Agostinho P, Cunha RA, Carvalho E, Fontes Ribeiro CA, Reis F, Pereira FC (2013) Spatial memory impairments in a prediabetic rat model. Neuroscience 250:565–577
Cellek S, Qu W, Schmidt AM, Moncada S (2004) Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: a new insight into selective nitrergic neuropathy in diabetes. Diabetologia 47:331–339
Jeyabal PV, Kumar R, Gangula PR, Micci MA, Pasricha PJ (2008) Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol Motil 20:253–261
Seftel AD, Vaziri ND, Ni Z, Razmjouei K, Fogarty J, Hampel N, Polak J, Wang RZ, Ferguson K, Block C, Haas C (1997) Advanced glycation end products in human penis: elevation in diabetic tissue, site of deposition, and possible effect through iNOS and eNOS. Urology 50:1016–1026
Komers R, Anderson S (2003) Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Ren Physiol 284:1121–1137
Korenaga K, Micci MA, Taglialatela G, Pasricha PJ (2006) Supression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil 18:392–400
Lai YL, Aoyama S, Nagai R, Miyoshi N, Ohshima H (2010) Inhibition of l-arginine metabolizing enzymes by l-arginine-derived advanced glycation end products. J Clin Biochem Nutr 46:177–185
Voukali E, Shotton HR, Lincoln J (2011) Selective responses of myenteric neurons to oxidative stress and diabetic stimuli. Neurogastroenterol Motil 23:964-e411. doi:10.1111/j.1365-2982.2011.01778.x
Wu F, Feng JZ, Qiu YH, Yu FB, Zhang JZ, Zhou W, Yu F, Wang GK, An LN, Ni FH, Wu H, Zhao XX, Qin YW, Luo HD (2013) Activation of receptor for advanced glycation end products contributes to aortic remodeling and endothelial dysfunction in sinoaortic denervated rats. Atherosclerosis 229:287–294
Lüth HJ, Ogunlade V, Kuhla B, Kientsch-Engel R, Stahl P, Webster J, Arendt T, Münch G (2005) Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb Cortex 15:211–220
Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G (2011) Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol Aging 32:763–777
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Shimazawa M, Inokuchi Y, Okuno T, Nakajima Y, Sakaguchi G, Kato A, Oku H, Sugiyama T, Kudo T, Ikeda T, Takeda M, Hara H (2008) Reduced retinal function in amyloid precursor protein-overexpressing transgenic mice via attenuating glutamate-N-methyl-d-aspartate receptor signaling. J Neurochem 107:279–290
Klingner M, Apelt J, Kumar A, Sorger D, Sabri O, Steinbach J, Scheunemann M, Schliebs R (2003) Alterations in cholinergic and non-cholinergic neurotransmitter receptor densities in transgenic Tg2576 mouse brain with β-amyloid plaque pathology. Int J Dev Neurosci 21:357–369
Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ (2010) Aβ-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci 30:5948–5957
Rodrigo J, Fernández-Vizarra P, Castro-Blanco S, Bentura ML, Nieto M, Gómez-Isla T, Martínez-Murillo R, Martínez A, Serrano J, Fernández AP (2004) Nitric oxide in the cerebral cortex of amyloid-precursor protein (SW)Tg2576 trangenic mice. Neuroscience 128:73–89
Martin BL, Tokheim AM, McCarthy PT, Doms BS, Davis AA, Armitage IM (2006) Metallothionein-3 and neuronal nitric oxide synthase levels in brains from the Tg2576 mouse model of Alzheimer’s disease. Mol Cell Biochem 283:129–137
Hartlage-Rübsamen M, Apelt J, Schliebs R (2001) Fibrillary beta-amyloid deposits are closely associated with atropic nitric oxide synthase (NOS)-expressing neurons but do not upregulate the inducible NOS in transgenic Tg2576 mouse brain with Alzheimer pathology. Neurosci Lett 302:73–76
Apelt J, Bigl M, Wunderlich P, Schliebs R (2004) Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int J Dev Neurosci 22:475–484
D’Uscio LV, Das P, Santhanam AVR, He T, Younkin SG, Katusic ZS (2012) Activation of PPARδ prevents endothelial dysfunction induced by overexpression of amyloid-β precursor protein. Cardiovasc Res 96:504–512
Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C (2002) Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol 283:H315–H323
Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H (1993) Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA 90:6434–6438
Chang JB, Chu NF, Syu JT, Hsieh AT, Hung YR (2011) Advanced glycation end products (AGEs) in relation to atherosclerotic lipid profiles in middle-aged and elderly diabetic patients. Lipids Health Dis 10:228. doi:10.1186/1476-511X-10-228
Lubitz I, Ricny J, Baranes D, Shemesh C, Kravitz E, Liraz-Zaltsman S, Maksin-Matveev A, Cooper I, Leibowitz A, Uribarri J, Schmeidler J, Kristofikova Z, Ripova D, LeRoith D, Schnaider-Beeri M High dietary advanced glycation end products impair spatial learning and accelerate Aβ deposition in an Alzheimer mouse model. Aging Cell (in review)
Fenaille F, Mottier P, Turesky RJ, Ali S, Guy PA (2001) Comparison of analytical techniques to quantify malondialdehyde in mild powders. J Chromatogr A 921:237–245
Agarwal R, Chase SD (2002) Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B Anal Technol Biomed Life Sci 775:121–126
Collingridge GL, Volianskis A, Bannister N, France G, Hanna L, Mercier M, Tidball P, Fang G, Irvine MW, Costa BM, Monaghan DT, Bortolotto ZA, Molnár E, Lodge D, Jane DE (2013) The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 64:13–26
West RK, Moshier E, Lubitz I, Schmeidler J, Godbold J, Cai W, Uribarri J, Vlassara H, Silverman J, Beeri MS (2014) Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech Ageing Dev 140:10–12
Wang L, Chen K, Liu K, Zhou Y, Zhang T, Wang B, Mi M (2015) DHA inhibited AGEs-induced retinal microglia activation via suppression of the PPARγ/NFκB pathway and reduction of signal transducers in the AGEs/RAGE axis recruitment into lipid rafts. Neurochem Res. doi:10.1007/s11064-015-1517-1
Guo HB, Cheng YF, Wu JG, Wang CM, Wang HT, Zhang C, Qiu ZK, Xu JP (2015) Donepezil improves learning and memory deficits in APP/PS1 mice by inhibition of microglial activation. Neuroscience. doi:10.1016/j.neuroscience.2015.01.058
Acknowledgments
The research was supported by Ministry of Education, Youth and Sports of the Czech Republic (Project KONTAKT II-LH13254) and by Grant Agency of the Czech Republic (Project P304/12/6069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in the study were in accordance with the ethical standards and were approved by the Institutional Animal Care and Use Committee of the Sheba Medical Center.
Rights and permissions
About this article
Cite this article
Kristofikova, Z., Ricny, J., Sirova, J. et al. Differences Between Tg2576 and Wild Type Mice in the NMDA Receptor–Nitric Oxide Pathway After Prolonged Application of a Diet High in Advanced Glycation End Products. Neurochem Res 40, 1709–1718 (2015). https://doi.org/10.1007/s11064-015-1654-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1654-6