Abstract
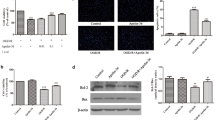
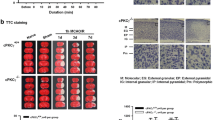
The adipocytokine apelin is a peptide, Apelin and its receptor are abundantly expressed in the nervous and cardiovascular systems. Previous studies had found apelin-13 reduces brain injuries and postischemic cerebral edema through blocking programmed cell death, Apelin-13 is also able to inhibit glucose deprivation induced cardiomyocyte autophagy in a concentration dependent fashion. To observe the effect of Apelin-13 on the brain injury induced by traumatic brain injury (TBI), and explore the effect of Apelin-13 on autophagy in TBI, We performed The neurological test, and the numbers of TBI-induced neural cell death were also counted by propidium iodide labeling. At last, the autophagy associated proteins LC3, Beclin-1, Bcl-2, p62 were also assessed with western-blotting. Compared with saline vehicle groups, the neural cell death, lesion volume, and neural dysfunction were attenuated by apelin-13 after TBI. In additionally, Apelin-13 also reversed TBI induced downregulation of LC3, Beclin-1, Bcl-2, p62 expression, compared with saline vehicle groups, at 24 and 48 h post TBI. Apelin-13 attenuates TBI induced brain damage by suppressing autophagy. All these results revealed that Apelin-13 suppressed autophagy. The autophagy may be involved in the mechanism of Apelin-13 rescue the subsequent damaged neuron in TBI.








Similar content being viewed by others
Abbreviations
- TBI:
-
Traumatic brain injury
- CCI:
-
Contolled cortical impact
- LC3:
-
Microtubule-associated protein 1 light chain 3
- PI:
-
Propidium iodide
References
Tatemoto K, Hosoya M, Habata Y et al (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251:471–476
O’Dowd BF, Heiber M, Chan A et al (1993) A human gene that shoos identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136:355–360
Simpkin JC, Yellon DM, Davidson SM et al (2007) Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia–reperfusion injury. Basic Res Cardiol 102:518–528
Kawamata Y, Habata Y, Fukusumi S et al (2001) Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta Mol Cell Res 1538:162–171
Hosoya M, Kawamata Y, Fukusumi S et al (2000) Molecular and functional characteristics of APJ. J Biol Chem 275:21061–21067
Susaki E, Wang G, Cao G et al (2005) Apelin cells in the rat stomach. Regul Pept 129:37–41
Choe W, Albright A, Sulcove J et al (2000) Functional expression of the seventransmembrane. J Neurovirol 6:S61–S69
Zeng XJ, Yu SP, Zhang L, Wei L (2010) Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res 316:1773–1783
O’Donnell LA, Agrawal A, Sabnekar P et al (2007) Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem 102:1905–1917
Khaksari M, Aboutaleb N, Nasirinezhad F et al (2012) Apelin-13 protects the brain against ischemic reperfusion injury and cerebral edema in a transient model of focal cerebral ischemia. J Mol Neurosci 48:201–208
Hui J, Zhi Z, Qinghua MA et al (2013) Mechanism underlying the inhibitory effect of Apelin-13 on glucose deprivation induced autophagy in rat cardiomyocytes. Exp Ther Med 5:797–802
Clark RS, Bayir H, Chu CT et al (2008) Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy 4(1):88–90
Bao Hai Jun, Wang T, Yang Tao Lu et al (2012) Poloxamer-188 attenuates TBI-induced blood-brain barrier damage leading to decreased brain edema and reduced cellular death. Neurochem Res 12:2856–2867
Luo CL, Li BX, Tao LY et al (2011) Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience 184:54–63
Pozuelo RM (2011) 14-3-3ζ binds class III phosphatidylinositol-3-kinase and inhibits autophagy. Autophagy 7:240–242
Liu CL, Chen S, Dietrich D et al (2008) Changes in autophagy after traumatic brain injury. J Cereb Blood Flow Metab 28:674–683
Ichimura Y, Kumanomidou T, Sou YS et al (2008) Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem 283:22847–22857
Lamark T, Kirkin V, Dikic I et al (2009) NBR1 and p62 as cargoreceptors for selective autophagy of ubiquitinated targets. Cell Cycle 8:1986–1990
Komatsu M, Ichimura Y (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 584:1374–1378
Pankiv S, Clausen TH, Lamark T et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Zheng YT, Shahnazari S, Brech A et al (2009) The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol 183:5909–5916
Qin ZH, Chen RW, Wang Y et al (1999) Nuclear factor kappaB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor-mediated apoptosis in rat striatum. J Neurosci 19:4023–4033
Feeney DM, Boyeson MG, Linnn RT et al (1981) Responses to cortical injury: I. Methodology and local effects of contusion in the rat. Brain Res 211:67–77
Whalen MJ, Dalkara T, You Z et al (2008) Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J Cereb Blood Flow Metab 28:490–450
Mbye LH, Keles E, Tao L et al (2012) Kollidon VA64, a membrane-resealing agent, reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 32:515–524
Ogata M (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Pattingre S, Levine B (2006) Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res 66:2885–2888
Pattingre S, Tassa A, Qu X et al (2005) Bcl-2 antiapoptotic proteins inhibit beclin-1 dependent Autophagy. Cell 122:927–939
Shankar S (2008) Changes in autophagy proteins in a rat model of controlled cortical imact induced brain injury. Biochem Biophys Res Commun 373:478–481
Mizushima N (2004) Methods for monitoring autophagy. Int J Biochem Cell Biol 36:2491–2502
Mizushima N, Yamamoto A, Matsui M et al (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Wei Y, Pattingre S, Sinha S et al (2008) NK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30:678–688
Bjørkøy G, Lamark T, Brech A (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Komatsu M, Wang QJ, Holstein GR et al (2007) Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A 104:14489–14494
Komatsu M, Kurokawa H, Waguri S et al (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12:213–223
Jain A, Lamark T, Sjøttem E et al (2010) p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response elementdriven gene transcription. J Biol Chem 285:22576–22591
Norman JM, Cohen GM, Bampton ET et al (2010) The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy 6:1042–1056
Daniel JK, Fabio CA, Hagai A et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Craig MS, Yaming C, Mara LS et al (2011) Autophagy in acute brain injury: feast, famine, or folly? Neurobiol Dis 43:52–59
Nagley P, Higgins GC, Atkin JD et al (2010) Multifaceted deaths orchestrated by mitochondria in neurones. Biochim Biophys Acta 1802:167–185
Puyal J, Peter GH, Clarke P et al (2009) Targeting autophagy to prevent neonatal stroke damage. Autophagy 5:1060–1061
Acknowledgments
The work was supported by the National Science Foundation of China (No 81302612).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hai-Jun Bao and Lin Zhang have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Bao, HJ., Zhang, L., Han, WC. et al. Apelin-13 Attenuates Traumatic Brain Injury-Induced Damage by Suppressing Autophagy. Neurochem Res 40, 89–97 (2015). https://doi.org/10.1007/s11064-014-1469-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1469-x