Abstract
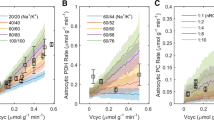
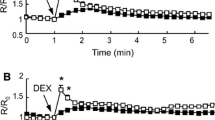
Neuronal excitation leads to an increase of the extracellular K+ concentration ([K+]o) in brain. This increase has at least two energy-consuming consequences: (1) a depolarization-mediated change in intracellular pH (pHi) in astrocytes due to depolarization-mediated increased activity of the acid-extruding Na+/bicarbonate transporter NBCe1 (driven by secondary active transport, supported by ion gradients established by the Na+, K+-ATPase); and (2) activation of cellular reuptake of K+ mediated by the Na+, K+-ATPase in both neurons and astrocytes. Astrocytic, but not neuronal increase in NBCe1 activity and pHi is also seen after chronic treatment with either of the two anti-bipolar drugs carbamazepine or valproic acid. The third ‘classical’ anti-bipolar drug, ‘lithium’ increases astrocytic pHi by a different mechanism (stimulation of the acid extruding Na+/H+ exchanger NHE1). The acid extruder fluxes, which depend upon the change in pHi per time unit (ΔpHi/Δt) and intracellular buffering power, have not been established in most of these situations. Therefore their stimulatory effects on energy metabolism has not been quantitated. This has been done in the present study in cultured mouse astrocytes. pHi was determined using the fluorescent pH-sensitive indicator BCECF–AM and an Olympus IX71 live cell imaging fluorescence microscope. Molar acid extrusion fluxes (indicating transporter activity) were determined as pHi changes/min during recovery after acid-loading with NH3/NH4 +, NBCe1 mRNA and protein expression in the cultured cells by, respectively RT-PCR and Western blotting. Drug-induced up-regulation of acid extrusion flux was slow and less than physiologically seen after increase in K+ concentration. Energetically, K+ uptake is much costlier than NBCe1 activity.








Similar content being viewed by others
References
Song D, Du T, Li B, Cai L, Gu L, Li H, Chen Y, Hertz L, Peng L (2008) Astrocytic alkalinization by therapeutically relevant lithium concentrations: implications for myo-inositol depletion. Psychopharmacology 200:187–195
Song D, Li B, Yan E, Man Y, Wolfson M, Chen Y, Peng L (2012) Chronic treatment with anti-bipolar drugs causes intracellular alkalinization in astrocytes, altering their functions. Neurochem Res 37:2524–2540
Lothman E, Lamanna J, Cordingley G, Rosenthal M, Somjen G (1975) Responses of electrical potential, potassium levels, and oxidative metabolic activity of the cerebral neocortex of cats. Brain Res 88:15–36
Krnjević K, Morris ME (1974) Extracellular accumulation of K+ evoked by activity of primary afferent fibers in the cuneate nucleus and dorsal horn of cats. Can J Physiol Pharmacol 52:852–871
Brookes N, Turner RJ (1994) K+-induced alkalinization in mouse cerebral astrocytes mediated by reversal of electrogenic Na+–HCO3 − cotransport. Am J Physiol 267:C1633–C1640
Chesler M, Kraig RP (1989) Intracellular pH transients of mammalian astrocytes. J Neurosci 9:2011–2019
Ransom CB, Ransom BR, Sontheimer H (2000) Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 522(Pt 3):427–442
Somjen GG, Kager H, Wadman WJ (2008) Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci 25:349–365
Macaulay N, Zeuthen T (2012) Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochem Res 37:2299–2309
Hertz L (1979) Inhibition by barbiturates of an intense net uptake of potassium into astrocytes. Neuropharmacology 18:629–632
Walz W, Hertz L (1982) Ouabain-sensitive and ouabain-resistant net uptake of potassium into astrocytes and neurons in primary cultures. J Neurochem 39:70–77
Peng L, Juurlink BH, Hertz L (1996) Pharmacological and developmental evidence that the potassium-induced stimulation of deoxyglucose uptake in astrocytes is a metabolic manifestation of increased Na(+)–K(+)-ATPase activity. Dev Neurosci 18(5–6):353–359
Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res 38:472–485
Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, Harkins LE, Boron WF, Roth KA, Bevensee MO (2008) Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience 155:818–832
Ruminot I, Gutiérrez R, Peña-Münzenmayer G, Añazco C, Sotelo-Hitschfeld T, Lerchundi R, Niemeyer MI, Shull GE, Barros LF (2011) NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+. J Neurosci 31:14264–14271
Walz W, Shargool M, Hertz L (1984) Barium-induced inhibition of K+ transport mechanisms in cortical astrocytes–its possible contribution to the large Ba2+-evoked extracellular K+ signal in brain. Neuroscience 13:945–949
Hertz L, Peng L, Lai JC (1998) Functional studies in cultured astrocytes. Methods 16:293–310
Hertz L (2012) Isotope-based quantitation of uptake, release, and metabolism of glutamate and glucose in cultured astrocytes. Methods Mol Biol 814:305–323
Li B, Zhang S, Li M, Zhang H, Hertz L, Peng L (2009) Down-regulation of GluK2 kainate receptor expression by chronic treatment with mood-stabilizing anti-convulsants or lithium in cultured astrocytes and brain, but not in neurons. Neuropharmacology 57:375–385
Meier E, Hertz L, Schousboe A (1991) Neurotransmitters as developmental signals. Neurochem Int 19:1–15
Rink TJ, Tsien RY, Pozzan T (1982) Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol 95:189–196
Grant RL, Acosta D (1997) Ratiometric measurement of intracellular pH of cultured cells with BCECF in a fluorescence multi-well plate reader. In Vitro Cell Dev Biol Anim 33:256–260
Manning TJ Jr, Sontheimer H (1999) Recording of intracellular Ca2+, Cl−, pH and membrane potential in cultured astrocytes using a fluorescence plate reader. J Neurosci Methods 91:73–81
Henderson PJ, McGivan JD, Chappell JB (1969) The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J 111:521–535
Thomas JA, Buchsbaum RN, Zimniak A, Racker E (1979) Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18:2210–2218
McAlear SD, Bevensee MO (2004) pH Regulation in non-neuronal brain cells and interstitial fluid. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 707–745
Boron WF, De Weer P (1976) Active proton transport stimulated by CO2/HCO3 −, blocked by cyanide. Nature 259:240–241
Bevensee MO, Apkon M, Boron WF (1997) Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J Gen Physiol 110:467–483
Kintner DB, Look A, Shull GE, Sun D (2005) Stimulation of astrocyte Na+/H+ exchange activity in response to in vitro ischemia depends in part on activation of ERK1/2. Am J Physiol Cell Physiol 289:C934–C945
McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM (2000) Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am J Physiol Cell Physiol 278:C676–C688
Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD (1992) Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol 458:361–384
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61:296–434
Nejsum LN, Praetorius J, Nielsen S (2005) NKCC1 and NHE1 are abundantly expressed in the basolateral plasma membrane of secretory coil cells in rat, mouse, and human sweat glands. Am J Physiol Cell Physiol 289:C333–C340
El Marjou M, Montalescot V, Buzyn A, Geny B (2000) Modifications in phospholipase D activity and isoform expression occur upon maturation and differentiation in vivo and in vitro in human myeloid cells. Leukemia 14:2118–2127
Li B, Hertz L, Peng L (2012) Aralar mRNA and protein levels in neurons and astrocytes freshly isolated from young and adult mouse brain and in maturing cultured astrocytes. Neurochem Int 61:1325–1332
Peng L, Martin-Vasallo P, Sweadner KJ (1997) Isoforms of Na, K-ATPase α and β subunits in the rat cerebellum and in granule cell cultures. J Neurosci 17:3488–3502
Chen Y, McNeill JR, Hajek I, Hertz L (1992) Effect of vasopressin on brain swelling at the cellular level: do astrocytes exhibit a furosemide–vasopressin-sensitive mechanism for volume regulation? Can J Physiol Pharmacol 70(Suppl):S367–S373
Østby I, Øyehaug L, Einevoll GT, Nagelhus EA, Plahte E, Zeuthen T, Lloyd CM, Ottersen OP, Omholt SW (2009) Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Comput Biol 5:e1000272
Florence CM, Baillie LD, Mulligan SJ (2012) Dynamic volume changes in astrocytes are an intrinsic phenomenon mediated by bicarbonate ion flux. PLoS One 7:e51124
Thomas RC (1972) Electrogenic sodium pump in nerve and muscle cells. Physiol Rev 52:563–594
Hamakawa H, Murashita J, Yamada N, Inubushi T, Kato N, Kato T (2004) Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci 58:82–88
Vadnal RE, Bazan NG (1988) Carbamazepine inhibits electroconvulsive shock-induced inositol trisphosphate (IP3) accumulation in rat cerebral cortex and hippocampus. Biochem Biophys Res Commun 153:128–134
Wu J, McNicholas CM, Bevensee MO (2009) Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci U S A 106:14150–14155
Thornell IM, Wu J, Liu X, Bevensee MO (2012) PIP2 hydrolysis stimulates the electrogenic Na+-bicarbonate cotransporter NBCe1-B and -C variants expressed in Xenopus laevis oocytes. J Physiol 590:5993–6011
Haque SK, Ariceta G, Batlle D (2012) Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant 27:4273–4287
Lee HJ, Park HJ, Lee S, Kim YH, Choi I (2011) The sodium-driven chloride/bicarbonate exchanger NDCBE in rat brain is upregulated by chronic metabolic acidosis. Brain Res 1377:13–20
Acknowledgments
This study was supported by Grant No. 31171036 from the National Natural Science Foundation of China.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dan Song and Yi Man have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Song, D., Man, Y., Li, B. et al. Comparison between drug-induced and K+-induced changes in molar acid extrusion fluxes (JH +) and in energy consumption rates in astrocytes. Neurochem Res 38, 2364–2374 (2013). https://doi.org/10.1007/s11064-013-1149-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1149-2