Abstract
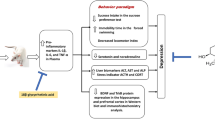
Impairment of neuroendocrine, immune and antioxidant defenses contribute to pathophysiology of stress-induced depression. Somatostatin executes diverse regulatory effects on endocrine, exocrine and neural functions; however, the possibility that octreotide, a synthetic somatostatin analogue might mitigate stress-induced depression remains elusive. Hence, the current study aimed to explore the immunomodulatory and antioxidant effects of octreotide in a model of chronic mild stress (CMS). This paradigm was performed by exposing rats to a combination of mild unpredictable stressors for 21 days. Fifty male Wistar rats were divided into five groups; (1) control receiving saline, (2) octreotide given to normal unstressed animals. The remaining three groups were subjected to (3) CMS alone or in combination with octreotide (4) 50 μg/kg or (5) 90 μg/kg. Octreotide increased sucrose preference index and attenuated CMS-induced increases in plasma adrenocorticotrophic hormone and corticosterone levels. In addition, octreotide decreased plasma tumor necrosis factor-alpha concentration. Moreover, it prevented CMS-induced oxidative damage by enhancing the antioxidant defenses superoxide dismutase, glutathione reductase and glutathione in the hippocampus. Furthermore, octreotide normalized the elevated malondialdehyde and lactate dehydrogenase levels in the hippocampus. These results demonstrate a possible antidepressant-like activity of octreotide in CMS due to its antioxidant/antiinflammatory aptitude.


Similar content being viewed by others
Abbreviations
- ACTH:
-
Adrenocorticotrophic hormone
- CMS:
-
Chronic mild stress
- CORT:
-
Corticosterone
- CRF:
-
Corticotrophin-releasing factor
- CRH:
-
Corticotrophin-releasing hormone
- DTNB:
-
5,5′-dithiobis-(2-nitrobenzoic acid)
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- HPA:
-
Hypothalamic-pituitary–adrenal axis
- LDH:
-
Lactate dehydrogenase
- MDA:
-
Malondialdehyde
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NADP:
-
Nicotinamide adenine dinucleotide
- NF-KB:
-
Nuclear factor-kappa B
- OCT:
-
Octreotide
- PVN:
-
Paraventricular nucleus
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- SP:
-
Sucrose preference
- SRIF:
-
Somatotropin release inhibiting factor
- TBA:
-
Trichloroacetic acid-thiobarbituric acid
- TNF-α:
-
Tumor necrosis factor-alpha
References
Kessler RC et al (1994) Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51(1):8–19
Johnson BN, Yamamoto BK (2009) Chronic unpredictable stress augments +3, 4-methylenedioxymethamphetamine-induced monoamine depletions: the role of corticosterone. Neuroscience 159(4):1233–1243
Tsigos C, Chrousos GP (2002) Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53(4):865–871
Lucca G et al (2009) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54(5–6):358–362
Lucca G et al (2009) Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res 43(9):864–869
Hayley S et al (2001) Central monoamine and plasma corticosterone changes induced by a bacterial endotoxin: sensitization and cross-sensitization effects. Eur J Neurosci 13(6):1155–1165
Maes M et al (1995) Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord 34(4):301–309
ter Veld F et al (2009) Effects of somatostatin and octreotide on cytokine and chemokine production by lipopolysaccharide-activated peripheral blood mononuclear cells. J Endocrinol Invest 32(2):123–129
Van Op den bosch J, Van Nassauw L, Van Marck E, Timmermans JP (2009) Somatostatin modulates mast cell-induced responses in murine spinal neurons and satellite cells. Am J Physiol Gastrointest Liver Physiol 297(2):G406–G417
Cervia D, Bagnoli P (2007) An update on somatostatin receptor signaling in native systems and new insights on their pathophysiology. Pharmacol Ther 116(2):322–341
Zhang K et al (1999) Decreased expression of the mRNA for somatostatin in the periventricular nucleus of depression-model rats. Life Sci 65(9):PL87–PL94
Lamers CB, Bijlstra AM, Harris AG (1993) Octreotide, a long-acting somatostatin analog, in the management of postoperative dumping syndrome. An update. Dig Dis Sci 38(2):359–364
Willner P (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134(4):319–329
Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16(4):525–534
Loas G (1996) Vulnerability to depression: a model centered on anhedonia. J Affect Disord 41(1):39–53
Erol FS et al (2008) Comparison of the effects of octreotide and melatonin in preventing nerve injury in rats with experimental spinal cord injury. J Clin Neurosci 15(7):784–790
Lai HS, Chen Y (1996) Effect of octreotide on postoperative intraperitoneal adhesions in rats. Scand J Gastroenterol 31(7):678–681
Grippo AJ et al (2005) Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav 84(5):697–706
Willner P et al (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93(3):358–364
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Schurr A, Livne A (1976) Differential inhibition of mitochondrial monoamine oxidase from brain by hashish components. Biochem Pharmacol 25(10):1201–1203
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Akerboom TP, Sies H (1981) Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol 77:373–382
Lowry OH et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Anacker C et al (2010) The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. Apr 15. [Epub ahead of print]
Maniam J, Morris MJ (2010) Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: role of hippocampus. Psychoneuroendocrinology (Jun 29. [Epub ahead of print])
Li S et al (2008) Chronic mild stress impairs cognition in mice: from brain homeostasis to behavior. Life Sci 82(17–18):934–942
Grippo AJ et al (2003) Cytokine mediation of experimental heart failure-induced anhedonia. Am J Physiol Regul Integr Comp Physiol 284(3):R666–R673
d’Audiffret AC et al (2010) Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol 108(5):1041–1051
Miron SD, Markowitz D, Minotti A (1998) Accumulation of In-111 Octreotide in brain infarcts: a case report. Clin Nucl Med 23(3):163–164
Peluso G et al (1996) Modulation of cytokine production in activated human monocytes by somatostatin. Neuropeptides 30(5):443–451
Eliakim R et al (1993) Octreotide effectively decreases mucosal damage in experimental colitis. Gut 34(2):264–269
Lang A et al (2005) Somatostatin inhibits pro-inflammatory cytokine secretion from rat hepatic stellate cells. Liver Int 25(4):808–816
Zhao J et al (2009) Insulin-like growth factor-I reduces stress-induced gastric mucosal injury by inhibiting neutrophil activation in mice. Growth Horm IGF Res 19(2):136–145
You JM et al (2009) Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can J Physiol Pharmacol 87(6):440–447
Filipovic D, Pajovic SB (2009) Differential regulation of CuZnSOD expression in rat brain by acute and/or chronic stress. Cell Mol Neurobiol 29(5):673–681
Djordjevic A et al (2010) Chronic social isolation suppresses proplastic response and promotes proapoptotic signalling in prefrontal cortex of Wistar rats. J Neurosci 88(11):2524–2533
Madrigal JL et al (2003) Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology 28(9):1579–1588
Olivenza R et al (2000) Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochem 74(2):785–791
Campo GM et al (2005) Purified human plasma glycosaminoglycans limit oxidative injury induced by iron plus ascorbate in skin fibroblast cultures. Toxicol In Vitro 19(5):561–572
Ahmad A et al (2010) Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress 13(4):355–364
Srikant CB, Patel YC (1981) Receptor binding of somatostatin-28 is tissue specific. Nature 294(5838):259–260
Fernandez-Checa JC et al (2010) Oxidative stress and altered mitochondrial function in neurodegenerative diseases: lessons from mouse models. CNS Neurol Disord Drug Targets 9(4):439–454
Kara H et al (2010) Protective effect of octreotide on intra-tracheal bleomycin-induced oxidative damage in rats. Exp Toxicol Pathol 62(3):235–241
Niedermuhlbichler M, Wiedermann CJ (1992) Suppression of superoxide release from human monocytes by somatostatin-related peptides. Regul Pept 41(1):39–47
Palsamy P, Sivakumar S, Subramanian S (2010) Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact 186(2):200–210
Deshmukh R et al (2009) Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine–a PDE1 inhibitor. Eur J Pharmacol 620(1–3):49–56
Celebi S et al (2002) Effects of melatonin, vitamin E and octreotide on lipid peroxidation during ischemia-reperfusion in the guinea pig retina. Eur J Ophthalmol 12(2):77–83
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaalan, M.F., Nassar, N.N. Effects of Octreotide in Chronically Mild Stressed Rats: Possible Role of Immune and Oxidative Stress Pathways. Neurochem Res 36, 1717–1723 (2011). https://doi.org/10.1007/s11064-011-0486-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0486-2