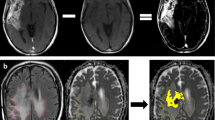
Abstract
Purpose
Autologous tumor lysate-loaded dendritic cell vaccine (DCVax-L) is a promising treatment modality for glioblastomas. The purpose of this study was to investigate the potential utility of multiparametric MRI-based prediction model in evaluating treatment response in glioblastoma patients treated with DCVax-L.
Methods
Seventeen glioblastoma patients treated with standard-of-care therapy + DCVax-L were included. When tumor progression (TP) was suspected and repeat surgery was being contemplated, we sought to ascertain the number of cases correctly classified as TP + mixed response or pseudoprogression (PsP) from multiparametric MRI-based prediction model using histopathology/mRANO criteria as ground truth. Multiparametric MRI model consisted of predictive probabilities (PP) of tumor progression computed from diffusion and perfusion MRI-derived parameters. A comparison of overall survival (OS) was performed between patients treated with standard-of-care therapy + DCVax-L and standard-of-care therapy alone (external controls). Additionally, Kaplan–Meier analyses were performed to compare OS between two groups of patients using PsP, Ki-67, and MGMT promoter methylation status as stratification variables.
Results
Multiparametric MRI model correctly predicted TP + mixed response in 72.7% of cases (8/11) and PsP in 83.3% (5/6) with an overall concordance rate of 76.5% with final diagnosis as determined by histopathology/mRANO criteria. There was a significant concordant correlation coefficient between PP values and histopathology/mRANO criteria (r = 0.54; p = 0.026). DCVax-L-treated patients had significantly prolonged OS than those treated with standard-of-care therapy (22.38 ± 12.8 vs. 13.8 ± 9.5 months, p = 0.040). Additionally, glioblastomas with PsP, MGMT promoter methylation status, and Ki-67 values below median had longer OS than their counterparts.
Conclusion
Multiparametric MRI-based prediction model can assess treatment response to DCVax-L in patients with glioblastoma.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CL:
-
Coefficient of linear anisotropy
- CP:
-
Coefficient of planar anisotropy
- CS:
-
Coefficient of spherical anisotropy
- CAR-T:
-
Chimeric antigen receptor T-cell
- CCRT:
-
Concurrent chemoradiation therapy
- DCVax-L:
-
Autologous tumor lysate-pulsed dendritic cell vaccine
- DTI:
-
Diffusion tensor imaging
- DSC:
-
Dynamic susceptibility contrast
- FA:
-
Fractional anisotropy
- GBM:
-
Glioblastoma
- IDH :
-
Isocitrate dehydrogenase
- MD:
-
Mean diffusivity
- MGMT:
-
O6-methylguanine-DNA-methyltransferase
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- PP:
-
Predictive probability of tumor progression
- PsP:
-
Pseudoprogression
- rCBV:
-
Relative cerebral blood volume
- SOC:
-
Standard-of-care
- TMZ:
-
Temozolomide
- TP:
-
True progression
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM (2020) Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep 10(1):11622
Montoya ML, Kasahara N, Okada H (2020) Introduction to immunotherapy for brain tumor patients: challenges and future perspectives. Neurooncol Pract 7:465–476
Desland FA, Hormigo A (2020) The CNS and the brain tumor microenvironment: implications for glioblastoma immunotherapy. Int J Mol Sci. https://doi.org/10.3390/ijms21197358
Boussiotis VA, Charest A (2018) Immunotherapies for malignant glioma. Oncogene 37:1121–1141
Prins RM, Craft N, Bruhn KW et al (2006) The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol 176:157–164
Prins RM, Soto H, Konkankit V et al (2011) Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res 17:1603–1615
Lepski G, Bergami-Santos PC, Pinho MP et al (2023) Adjuvant vaccination with allogenic dendritic cells significantly prolongs overall survival in high-grade gliomas: results of a phase II trial. Cancers. https://doi.org/10.3390/cancers15041239
Liau LM, Ashkan K, Tran DD et al (2018) First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med 16:142
Liau LM, Ashkan K, Brem S et al (2022) Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma. JAMA Oncol 9:112–121
Chawla S, Shehu V, Gupta PK et al (2021) Physiological imaging methods for evaluating response to immunotherapies in glioblastomas. Int J Mol Sci 22:3867
da Cruz LCH, da Cruz LCH, Rodriguez I et al (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiol 32:1978–1985
Okada H, Weller M, Huang R et al (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16:e534–e542
Ellingson BM, Wen PY, Cloughesy TF (2017) Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 14:307–320
Qin L, Li X, Stroiney A et al (2017) Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma. Neuroradiology 59:135–145
Song J, Kadaba P, Kravitz A et al (2020) Multiparametric MRI for early identification of therapeutic response in recurrent glioblastoma treated with immune checkpoint inhibitors. Neuro Oncol 22:1658–1666
Wang S, O’Rourke DM, Chawla S et al (2019) Multiparametric magnetic resonance imaging in the assessment of anti-EGFRvIII chimeric antigen receptor T cell therapy in patients with recurrent glioblastoma. Br J Cancer 120:54–56
Vrabec M, Van Cauter S, Himmelreich U et al (2011) MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology 53:721–731
Stenberg L, Englund E, Wirestam R et al (2006) Dynamic susceptibility contrast-enhanced perfusion magnetic resonance (MR) imaging combined with contrast-enhanced MR imaging in the follow-up of immunogene-treated glioblastoma multiforme. Acta radiol 47:852–861
Wang S, Martinez-Lage M, Sakai Y et al (2016) Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. AJNR Am J Neuroradiol 37:28–36
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251
Verma G, Chawla S, Mohan S et al (2019) Three-dimensional echo planar spectroscopic imaging for differentiation of true progression from pseudoprogression in patients with glioblastoma. NMR Biomed 32:e4042
Bagley SJ, Schwab RD, Nelson E et al (2019) Histopathologic quantification of viable tumor versus treatment effect in surgically resected recurrent glioblastoma. J Neurooncol 141:421–429
Akbari H, Rathore S, Bakas S et al (2020) Histopathology-validated machine learning radiographic biomarker for noninvasive discrimination between true progression and pseudo-progression in glioblastoma. Cancer 126:2625–2636
Mohan S, Wang S, Coban G et al (2019) Detection of occult neoplastic infiltration in the corpus callosum and prediction of overall survival in patients with glioblastoma using diffusion tensor imaging. Eur J Radiol 112:106–111
Rahman R, Ventz S, McDunn J et al (2021) Leveraging external data in the design and analysis of clinical trials in neuro-oncology. Lancet Oncol 22:e456–e465
Wang S, Kim S, Chawla S et al (2009) Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage 44:653–660
Chawla S, Wang S, Mohan S et al (2019) Differentiation of brain infection from necrotic glioblastoma using combined analysis of diffusion and perfusion MRI. J Magn Reson Imaging 49:184–194
Nasrallah MP, Binder ZA, Oldridge DA et al (2019) Molecular neuropathology in practice: clinical profiling and integrative analysis of molecular alterations in glioblastoma. Acad Pathol 6:2374289519848353
Kasten BB, Udayakumar N, Leavenworth JW et al (2019) Current and future imaging methods for evaluating response to immunotherapy in neuro-oncology. Theranostics 9:5085–5104
Liau LM, Prins RM, Kiertscher SM et al (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11:5515–5525
Ellingson BM, Sampson J, Achrol AS et al (2021) Modified RANO, immunotherapy RANO, and standard RANO response to convection-enhanced delivery of IL4R-targeted immunotoxin MDNA55 in recurrent glioblastoma. Clin Cancer Res 27:3916–3925
Heugenhauser J, Galijasevic M, Mangesius S et al (2022) MRI response assessment in glioblastoma patients treated with dendritic-cell-based immunotherapy. Cancers. https://doi.org/10.3390/cancers14061579
Aquino D, Gioppo A, Finocchiaro G et al (2017) MRI in glioma immunotherapy: evidence, pitfalls, and perspectives. J Immunol Res 2017:5813951
Rowe LS, Butman JA, Mackey M et al (2018) Differentiating pseudoprogression from true progression: analysis of radiographic, biologic, and clinical clues in GBM. J Neurooncol 139:145–152
Zikou A, Sioka C, Alexiou GA et al (2018) Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Med Mol Imaging 2018:6828396
Mohan S, Wang S, Chawla S et al (2021) Multiparametric MRI assessment of response to convection-enhanced intratumoral delivery of MDNA55, an interleukin-4 receptor targeted immunotherapy, for recurrent glioblastoma. Surg Neurol Int 12:337
Gerstner ER, McNamara MB, Norden AD et al (2009) Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol 94:97–101
Gunjur A, Lau E, Taouk Y, Ryan G (2011) Early post-treatment pseudo-progression amongst glioblastoma multiforme patients treated with radiotherapy and temozolomide: a retrospective analysis. J Med Imaging Radiat Oncol 55:603–610
Kang H-C, Kim C-Y, Han JH et al (2011) Pseudoprogression in patients with malignant gliomas treated with concurrent temozolomide and radiotherapy: potential role of p53. J Neurooncol 102:157–162
Sanghera P, Perry J, Sahgal A et al (2010) Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 37:36–42
Van Mieghem E, Wozniak A, Geussens Y et al (2013) Defining pseudoprogression in glioblastoma multiforme. Eur J Neurol 20:1335–1341
Li H, Li J, Cheng G et al (2016) IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg 151:31–36
Weller M, Stupp R, Reifenberger G et al (2010) MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6:39–51
Mishra-Kalyani PS, Amiri Kordestani L, Rivera DR et al (2022) External control arms in oncology: current use and future directions. Ann Oncol 33:376–383
Acknowledgements
The support of Penn Neuroradiology Clinical Research Core (Ms. Lauren Karpf, Ms. Lisa Desiderio, and Dr. Shadi Asadollah) and Penn Neurosurgery Clinical Research Division (Ms. Eileen Maloney) and clinical research coordinators is gratefully acknowledged. The dendritic cell vaccine trial at the University of Pennsylvania was sponsored by Northwest Biotherapeutics, the protocol, “A Phase-3 Clinical Trial Evaluating DC Vax®L, Autologous Dendritic Cells Pulsed with Tumor Lysate Antigen for the Treatment of Glioblastoma Multiforme”, approved by the Abramson Cancer Center – CTSRMC (Clinical Trials Scientific Review Committee (UPCC # 34313) and the Institutional Review Board (#81817). The data analyses and the drafting of the manuscript were performed by the authors, independent of the sponsor.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Laiz Laura de Godoy: Conceptualization; Data curation; Investigation; Project administration; Writing—original draft; Writing—review & editing. Sanjeev Chawla: Conceptualization; Data curation; Investigation; Methodology; Supervision; Writing—original draft; Writing—review & editing. Steven Brem: Conceptualization; Investigation; Supervision; Writing—review & editing. Sumei Wang: Formal analysis; Methodology; Software; Writing—review & editing. Donald M. O’Rourke: Investigation; Writing—review & editing. MacLean P. Nasrallah: Data curation; Investigation; Writing—review & editing. Arati Desai: Investigation; Writing—review & editing. Laurie A. Loevner: Investigation; Writing—review & editing. Linda M. Liau: Conceptualization; Investigation; Writing—review & editing. Suyash Mohan: Conceptualization; Investigation; Methodology; Supervision; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
Dr. S. Brem received partial support of travel expenses in 2022 from Northwest Biotherapeutics. The remaining authors do not have any potential conflicts of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The DCVax-L trial at the University of Pennsylvania, “A Phase-3 Clinical Trial Evaluating DC Vax®L, Autologous Dendritic Cells Pulsed with Tumor Lysate Antigen for the Treatment of Glioblastoma Multiforme” was sponsored by Northwest Biotherapeutics, using the protocol, NCT00045968. It was approved by the Abramson Cancer Center—CTSRMC (Clinical Trials Scientific Review Committee (UPCC # 34313) and the Institutional Review Board (#81817).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Godoy, L.L., Chawla, S., Brem, S. et al. Assessment of treatment response to dendritic cell vaccine in patients with glioblastoma using a multiparametric MRI-based prediction model. J Neurooncol 163, 173–183 (2023). https://doi.org/10.1007/s11060-023-04324-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04324-4