Abstract
Purpose
Resting state functional magnetic resonance imaging (rsfMRI) is an emerging tool to explore the functional connectivity of different brain regions. We aimed to assess the disruption of functional connectivity of the Default Mode Network (DMN), Dorsal Attention Network(DAN) and Fronto-Parietal Network (FPN) in patients with glial tumors.
Methods
rsfMRI data acquired on 3T-MR of treatment-naive glioma patients prospectively recruited (2015–2019) and matched controls from the 1000 functional-connectomes-project were analyzed using the CONN functional toolbox. Seed-Based Connectivity Analysis (SBCA) and Independent Component Analysis (ICA, with 10 to 100 components) were performed to study reliably the three networks of interest.
Results
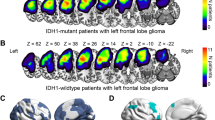
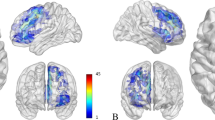
35 patients with gliomas (17 WHO grade I-II, 18 grade III-IV) and 70 controls were included. Global increased DMN connectivity was consistently found with SBCA and ICA in patients compared to controls (Cluster1: Precuneus, height: p < 10−6; Cluster2: subcallosum; height: p < 10−5). However, an area of decreased connectivity was found in the posterior corpus callosum, particularly in high-grade gliomas (height: p < 10−5). The DAN demonstrated small areas of increased connectivity in frontal and occipital regions (height: p < 10−6). For the FPN, increased connectivity was noted in the precuneus, posterior cingulate gyrus, and frontal cortex. No difference in the connectivity of the networks of interest was demonstrated between low- and high-grade gliomas, as well as when stratified by their IDH1-R132H (isocitrate dehydrogenase) mutation status.
Conclusion
Altered functional connectivity is reliably found with SBCA and ICA in the DMN, DAN, and FPN in glioma patients, possibly explained by decreased connectivity between the cerebral hemispheres across the corpus callosum due to disruption of the connections.


Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DAN:
-
Dorsal attention network
- DMN:
-
Default mode network
- FPN:
-
Fronto-parietal executive control network
- ICA:
-
Independent component analysis
- ICs:
-
Independent components
- IDH:
-
Isocitrate dehydrogenase
- rsfMRI:
-
Resting state functional magnetic resonance imaging
- SBCA:
-
Seed-based correlation analysis
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507. https://doi.org/10.1056/NEJMra0708126
Gould J (2018) Breaking down the epidemiology of brain cancer. Nature 561:S40–S41. https://doi.org/10.1038/d41586-018-06704-7
Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR (2019) Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 15:405–417. https://doi.org/10.1038/s41582-019-0220-2
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Conti Nibali M, Rossi M, Sciortino T, et al (2019) Preoperative surgical planning of glioma: limitations and reliability of fMRI and DTI tractography. J Neurosurg Sci 63:127–134. https://doi.org/10.23736/S0390-5616.18.04597-6
Ogawa S, Menon RS, Tank DW et al (1993) Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 64:803–812. https://doi.org/10.1016/S0006-3495(93)81441-3
Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87:9868–9872. https://doi.org/10.1073/pnas.87.24.9868
Lv H, Wang Z, Tong E et al (2018) Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol 39:1390–1399. https://doi.org/10.3174/ajnr.A5527
Crosson B, Ford A, McGregor KM et al (2010) Functional Imaging and related techniques: an introduction for rehabilitation researchers. J Rehabil Res Dev 47:vii–xxxiv
Raichle ME, MacLeod AM, Snyder AZ et al (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258. https://doi.org/10.1073/pnas.0135058100
Yan C-G, Chen X, Li L et al (2019) Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci U S A 116:9078–9083. https://doi.org/10.1073/pnas.1900390116
Guo X, Duan X, Suckling J et al (2019) Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Hum Brain Mapp 40:1264–1275. https://doi.org/10.1002/hbm.24447
Jiang L, Geng W, Chen H et al (2018) Decreased functional connectivity within the default-mode network in acute brainstem ischemic stroke. Eur J Radiol 105:221–226. https://doi.org/10.1016/j.ejrad.2018.06.018
Harris RJ, Bookheimer SY, Cloughesy TF et al (2014) Altered functional connectivity of the default mode network in diffuse gliomas measured with pseudo-resting state fMRI. J Neuro-Oncol 116:373–379. https://doi.org/10.1007/s11060-013-1304-2
Spreng RN, Sepulcre J, Turner GR et al (2013) Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci 25:74–86. https://doi.org/10.1162/jocn_a_00281
Dixon ML, Vega ADL, Mills C et al (2018) Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. PNAS 115:E1598–E1607. https://doi.org/10.1073/pnas.1715766115
Fox ME, King TZ (2018) Functional connectivity in adult brain tumor patients: a systematic review. Brain Connect 8:381–397. https://doi.org/10.1089/brain.2018.0623
Bennett C, Miller M, Wolford G (2009) Neural correlates of interspecies perspective taking in the post-mortem Atlantic Salmon: an argument for multiple comparisons correction. Neuroimage 47. https://doi.org/10.1016/S1053-8119(09)71202-9
Warren DE, Sutterer MJ, Bruss J et al (2017) Surgically disconnected temporal pole exhibits resting functional connectivity with remote brain regions. bioRxiv 127571. https://doi.org/10.1101/127571
Franco AR, Mannell MV, Calhoun VD, Mayer AR (2013) Impact of analysis methods on the reproducibility and reliability of resting-state networks. Brain Connect 3:363–374. https://doi.org/10.1089/brain.2012.0134
Metwali H, Samii A (2019) Seed-based connectivity analysis of resting-state fMRI in patients with brain Tumors: a feasibility study. World Neurosurg 128:e165–e176. https://doi.org/10.1016/j.wneu.2019.04.073
Liouta E, Katsaros VK, Stranjalis G et al (2019) Motor and language deficits correlate with resting state functional magnetic resonance imaging networks in patients with brain tumors. J Neuroradiol 46:199–206. https://doi.org/10.1016/j.neurad.2018.08.002
Mennes M, Biswal BB, Castellanos FX, Milham MP (2013) Making data sharing work: the FCP/INDI experience. NeuroImage 82:683–691. https://doi.org/10.1016/j.neuroimage.2012.10.064
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. https://doi.org/10.1089/brain.2012.0073
Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151
Peer M, Nitzan M, Bick AS et al (2017) Evidence for functional networks within the human Brain’s white matter. J Neurosci 37:6394–6407. https://doi.org/10.1523/JNEUROSCI.3872-16.2017
Gore JC, Li M, Gao Y et al (2019) Functional MRI and resting state connectivity in white matter - a mini-review. Magn Reson Imaging 63:1–11. https://doi.org/10.1016/j.mri.2019.07.017
Saksena S, Jain R, Schultz L et al (2013) The corpus callosum Wallerian degeneration in the unilateral brain tumors: evaluation with diffusion tensor imaging (DTI). J Clin Diagn Res 7:320–325. https://doi.org/10.7860/JCDR/2013/4491.2757
Cholet C, Leclercq D, Law-Ye B (2017) Crossing the line: brainstem lesion in a patient with glioblastoma. J Clin Neurosci 46. https://doi.org/10.1016/j.jocn.2017.08.058
de Lacoste MC, Kirkpatrick JB, Ross ED (1985) Topography of the human corpus callosum. J Neuropathol Exp Neurol 44:578–591. https://doi.org/10.1097/00005072-198511000-00004
Tantillo E, Vannini E, Cerri C et al (2019) Bidirectional neuron-glioma interactions: effects of glioma cells on synaptic activity and its impact on tumor growth. Neuro Oncol 21:iv1–iv1. https://doi.org/10.1093/neuonc/noz167.000
Venkatesh HS, Johung TB, Caretti V et al (2015) Neuronal activity promotes glioma growth through Neuroligin-3 secretion. Cell 161:803–816. https://doi.org/10.1016/j.cell.2015.04.012
Venkatesh HS, Morishita W, Geraghty AC et al (2019) Electrical and synaptic integration of glioma into neural circuits. Nature 573:539–545. https://doi.org/10.1038/s41586-019-1563-y
Sparacia G, Parla G, Lo Re V et al (2020) Resting-state functional connectome in patients with brain Tumors before and after surgical resection. World Neurosurg. https://doi.org/10.1016/j.wneu.2020.05.054
Liu D, Hu X, Liu Y et al (2019) Potential intra- or cross-network functional reorganization of the triple unifying networks in patients with frontal glioma. World Neurosurg 128:e732–e743. https://doi.org/10.1016/j.wneu.2019.04.248
Hart MG, Price SJ, Suckling J (2017) Functional connectivity networks for preoperative brain mapping in neurosurgery. J Neurosurg 126:1941–1950. https://doi.org/10.3171/2016.6.JNS1662
Böttger J, Margulies DS, Horn P et al (2011) A software tool for interactive exploration of intrinsic functional connectivity opens new perspectives for brain surgery. Acta Neurochir 153:1561–1572. https://doi.org/10.1007/s00701-011-0985-6
Rocca MA, Parisi L, Pagani E et al (2014) Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology 273:511–520. https://doi.org/10.1148/radiol.14140417
Mukherjee P, Bahn MM, McKinstry RC et al (2000) Differences between gray matter and white matter water diffusion in stroke: diffusion-tensor MR imaging in 12 patients. Radiology 215:211–220. https://doi.org/10.1148/radiology.215.1.r00ap29211
Price SJ, Allinson K, Liu H et al (2017) Less invasive phenotype found in Isocitrate dehydrogenase-mutated glioblastomas than in Isocitrate dehydrogenase wild-type glioblastomas: a diffusion-tensor imaging study. Radiology 283:215–221. https://doi.org/10.1148/radiol.2016152679
Suh CH, Kim HS, Jung SC, Kim SJ (2018) Diffusion-weighted imaging and diffusion tensor imaging for differentiating high-grade glioma from solitary brain metastasis: a systematic review and meta-analysis. AJNR Am J Neuroradiol 39:1208–1214. https://doi.org/10.3174/ajnr.A5650
Jütten K, Mainz V, Gauggel S et al (2019) Diffusion tensor imaging reveals microstructural heterogeneity of normal-appearing white matter and related cognitive dysfunction in glioma patients. Front Oncol 9:536. https://doi.org/10.3389/fonc.2019.00536
Cho NS, Jenabi M, Arevalo-Perez J et al (2018) Diffusion tensor imaging shows corpus callosum differences between high-grade gliomas and metastases. J Neuroimaging 28:199–205. https://doi.org/10.1111/jon.12478
Agcaoglu O, Wilson TW, Wang Y-P et al (2019) Resting state connectivity differences in eyes open versus eyes closed conditions. Hum Brain Mapp 40:2488–2498. https://doi.org/10.1002/hbm.24539
Tung K-C, Uh J, Mao D et al (2013) Alterations in resting functional connectivity due to recent motor task. Neuroimage 78:316–324. https://doi.org/10.1016/j.neuroimage.2013.04.006
Grigg O, Grady CL (2010) Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One 5:e13311. https://doi.org/10.1371/journal.pone.0013311
Funding
This work was supported by the following grants: NIH/ National Institute of Biomedical Imaging and Bioengineering: R01EB026456; NIH/ National Institute of Neurological Disorders and Stroke: R01NS097494; Agence Régionale de Santé Ile de France (MT).
Author information
Authors and Affiliations
Contributions
Conceptualization: Rajan Jain; Methodology: Mickael Tordjman, Guillaume Madelin, Mariana Lazar, Rajan Jain; Formal analysis and investigation: Mickael Tordjman, Pradeep Kumar Gupta, Christine Cordova, Sylvia C. Kurz, Daniel Orringer, John Golfinos, Douglas Kondziolka, Yulin Ge, Ruoyu Luie Wang; Writing - original draft preparation: Mickael Tordjman, Rajan Jain; Writing - review and editing: Guillaume Madelin, Christine Cordova, Sylvia C. Kurz, Daniel Orringer, John Golfinos, Douglas Kondziolka, Yulin Ge, Mariana Lazar, Rajan Jain.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Ethics approval
(Include appropriate approvals or waivers). This study was approved by the Institutional Review Board (IRB:i1700006).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tordjman, M., Madelin, G., Gupta, P.K. et al. Functional connectivity of the default mode, dorsal attention and fronto-parietal executive control networks in glial tumor patients. J Neurooncol 152, 347–355 (2021). https://doi.org/10.1007/s11060-021-03706-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03706-w