Abstract
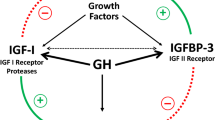
To assess the long-term safety of administering growth hormone (GH) in patients with GH deficiency due to treatment for childhood medulloblastoma and primitive neuroectodermal tumor (PNET). Data were retrospectively retrieved on children receiving GH supplementation, assessing their disease-free and overall survival outcomes and risk of secondary malignancies using Kaplan–Meier and Cox models. Overall 65 children were consecutively collected from May 1981 to April 2013. All patients had undergone craniospinal irradiation (total dose 18–39 Gy), and subsequently received GH for a median (interquartile range, IQR) of 81 (50.6–114.9) months. At a median (IQR) of 122.4 months (74.4–149.5) after the end of their adjuvant cancer treatment, two patients (3 %) experienced recurrent disease and 8 (12.3 %) developed secondary malignancies, all but one of them (an osteosarcoma) related to radiation exposure and occurring within the radiation fields. There was no apparent correlation between the administration of GH replacement therapy (or its duration) and primary tumor relapse or the onset of secondary malignancies [HR: 1.01 (95 % CI: 0.98, 1.03) for every additional 12 months of GH supplementation; p = 0.36). At univariate analysis, the large cell or anaplastic medulloblastoma subtype, metastases and myeloablative chemotherapy correlated with a higher risk of secondary malignancies (p < 0.1), but multivariate analysis failed to identify any factors independently associated with this risk. Our data supports once more the safety of long-term GH replacement therapy in children treated for medulloblastoma/PNET, previously reported in larger data sets. The neurooncology community now need to warrant large-scale meta-analyses or international prospective trials in order to consolidate our knowledge of factors other than GH, such as genetic predisposition, high-grade/metastatic disease, high-dose chemotherapy and era of treatment, in promoting the occurrence of secondary malignancies.


Similar content being viewed by others
References
Childhood Cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M et al. (eds) (2012) SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). National Cancer Institute, Bethesda
Smoll NR, Drummond KJ (2012) The incidence of medulloblastomas and primitive neuroectodermal tumours in adults and children. J Clin Neurosci 19(11):1541–1544
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Johnston DL, Keen DL, Lafay-Cousin L et al (2008) Supratentorial primitive neuroectodermal tumors: a Canadian pediatric brain tumor consortium report. J Neurooncol 86:101–108
Tong X, Deng X, Yang T et al (2015) Clinical presentation and long-term outcome of primary spinal peripheral primitive neuroectodermal tumors. J Neurooncol 124(3):455–463
Packer RJ, Gaijar A, Vezina G et al (2006) Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24(25):4202–4208
Gaijar A, Chintagumpala M, Ashley D et al (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7(10):813–820
Gandola L, Massimino M, Cefalo G et al (2009) Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol 27(4):557–566
Lannering B, Rutkowski S, Doz F et al (2012) Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 30(26):3187–3193
Massimino M, Gandola L, Biassoni V et al (2013) Evolving of therapeutic strategies for CNS-PNET. Pediatr Blood Cancer 60(12):2031–2035
Packer RJ, Zhou T, Holmes E et al (2013) Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro-Oncology 15(1):97–103
Gunn ME, Lähdesmäki T, Malila N et al (2016) Use of endocrinological and neurological medication among 5-year survivors of young onset brain tumors. J Neurooncol 128(3):473–479
Bull KS, Spoudeas HA, Yadegarfar G et al (2007) Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: a follow-up study in PNET 3 trial survivors—on behalf of the CCLG (formerly UKCCSG). J Clin Oncol 25(27):4239–4245
Armstrong JT, Liu Q, Yasui Y et al (2009) Long-term outcome among adult survivors of childhood central nervous system malignancies: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 101:1–12
Moxon-Emre I, Bouffet E, Taylor MD et al (2014) Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol 32(17):1760–1768
Gleeson HK, Shalet SM (2004) The impact of cancer therapy on the endocrine system in survivors of childhood brain tumors. Endocr Relat Cancer 11:589–602
Uday S, Murray RD, Picton S et al (2015) Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clin Endocrinol (Oxf) 83(5):663–670
Chemaitilly W, Sklar CA (2010) Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer 17(3):R141–159
Gan HW, Phipps K, Aquilina K et al (2015) Neuroendocrine morbidity after pediatric optic gliomas: a longitudinal analyses of 166 children over 30 years. J Clin Endocrinol Metab 100(10):3787–3799
Spoudeas HA, Charmandari E, Brook CGD (2003) Hypothalamo-pituitary-adrenal axis integrity after cranial irradiation for childhood posterior fossa tumors. Med Pediatr Oncol 40:224–229
Spoudeas HA (2002) Growth following malignancy. Best Pract Res Clin Endocrinol Metab 16(3):561–590
van Waas M, Neggers SJ, van der Lelij A-J et al (2010) The metabolic syndrome in adult survivors of childhood cancer, a review. J Pediatr Hematol Oncol 32:171–179
Quik EH, Valk GD, Drent ML et al (2012) Reduced growth hormone secretion contributes after cranial irradiation contributes to neurocognitive dysfunction. Growth Horm IGF Res 22(1):42–47
Filipsson H, Johannsson G (2009) GH replacement in adults: interactions with other pituitary hormone deficiencies and replacement therapies. Eur J Endocrinol 161(Suppl 1):S85–S95
Thomas JD, Monson JP (2009) Adult GH deficiency throughout lifetime. Eur J Endocrinol 161(Suppl 1):S97–S106
Blum WF, Schweizer R (2003) Insulin-like growth factors and their binding proteins. In: Ranke MB (ed). Diagnostics of Endocrine function in children and adolescents, pp 166–199
Tao Y, Pinzi V, Bourhis J, Deutsch E (2007) Mechanism of disease: signaling of the insulin-like growth factor 1 receptor pathway-therapeutic perspectives in cancer. Nat Clin Pract Oncol 4:591–602
Mackenzie S, Craven T, Gattamaneni HR et al (2011) Long-term safety of growth hormone replacement after CNS irradiation. J Clin Endocrinol Metab 96(9):2756–2761
Shen L, Sun CM, Li XT et al (2015) Growth hormone therapy and risk of recurrence/progression in intracranial tumors: a meta-analysis. Neurol Sci 36(10):1859–1867
Massimino M, Gandola L, Cefalo G et al (2000) Management of medulloblastoma and ependymoma in infants: a single-institution long-term retrospective report. Childs Nerv Syst 16(1):12–15
Fangusaro J, Massimino M, Rutkowski S et al (2010) Non-cerebellar primitive neuroectodermal tumors (PNET): summary of the Milan consensus and state of the art workshop on marrow ablative chemotherapy with hematopoietic cell rescue for malignant brain tumors of childhood and adolescents. Pediatr Blood Cancer 54(4):638–640
Massimino M, Cefalo G, Riva D et al (2012) Long-term results of combined preradiation chemotherapy and age-tailored radiotherapy doses for childhood medulloblastoma. J Neurooncol 108(1):163–171
Massimino M, Gandola L, Biassoni V et al (2013) Evolving of therapeutic strategies for CNS PNET. Pediatr Blood Cancer 60(12):2031–2035
Biassoni V, Pallotti F, Spreafico F et al (2009) A female survivor of childhood medulloblastoma presenting with growth-hormone-induced edema and inflammatory lesions: a case report. J Med Case Rep 16(3):17
Friend KE, Radinsky R, McCutcheon IE (1999) Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg 91(1):93–99
Friend KE, Khandwala HM, Flyvbjerg A et al (2001) Growth hormone and insulin-like growth factor-I: effects on the growth of glioma cell lines. Growth Horm IGF Res 11(2):84–91
Pekic S, Popovic V (2013) GH therapy and cancer risk in hypopituitarism: what we know from human studies. Eur J Endocrinol 169(5):R89–R97
Takahara K, Tearle H, Ghaffari M et al (2011) Human prostate cancer xenografts in lit/lit mice exhibit reduced growth and androgen-independent progression. Prostate 71:525–537
Park SL, Setiawan VW, Kanetsky PA et al (2011) Serum insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels with risk of malignant melanoma. Cancer Causes Control 22:1267–1275
Zhang R, Xu GL, Li Y et al (2013) The role of insulin-like growth factor 1 and its receptor in the formation and development of colorectal carcinoma. J Int Med Res 41(4):1228–1235
Price AJ, Allen NE, Appleby PN et al (2012) Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 21:1531–1541
Rostoker R, Abelson S, Bitton-Worms K et al (2015) Highly specific role of the insulin receptor in breast cancer progression. Endocr Relat Cancer 22(2):145–157
Mu N, Zhu Y, Wang Y et al (2012) Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol 125:751–757
Swerdlow AJ, Reddingius RE, Higgins CD et al (2003) Growth hormone treatment in children with brain tumors and risk of tumor recurrence. J Clin Endocrinol Metab 85(12):4444–4449
Raman S, Grimberg A, Waguespack SG et al (2015) Risk of neoplasia in pediatric patients receiving growth hormone therapy—a report from the Pediatric Endocrine Society Drug and Therapeutics Committee. J Clin Endocrinol Metab 100(6):2192–2203
Nishio S, Morioka T, Inamura T et al (1998) Radiation-induced brain tumours: potential late complications of radiation therapy for brain tumours. Acta Neurochir (Wien) 140:763–770
Brignardello E, Felicetti F, Castiglione A et al (2015) GH replacement therapy and second neoplasms in adult survivors of childhood cancer: a retrospective study from a single institution. J Endocrinol Invest 38(2):171–176
Ning MS, Perkins SM, Dewees T, Shinohara ET (2015) Evidence of high mortality in long term survivors of childhood medulloblastoma. J Neurooncol 122:321–327
Arnold JR, Arnold DF, Marland A et al (2009) GH replacement in patients with non-functioning pituitary adenoma (NFA) treated solely by sugery is not associated with increased risk of tumour recurrence. Clin Endocrinol (Oxf) 70(3):435–438
Olsson DS, Buchfelder M, Wiendieck K et al (2012) Tumour recurrence and enlargement in patients with craniopharyngioma with and without GH replacement therapy during more than 10 years of follow-up. Eur J Endocrinol 166(6):1061–1068
Shen L, Sun CM, Li XT et al (2015) Growth hormone therapy and risk of recurrence/progression in intracranial tumors: a meta-analysis. Neurol Sci 36:1859–1867
Patterson BC, Chen Y, Sklar CA et al (2014) Growth hormone exposure as a risk factor for the development of subsequent neoplasms of the central nervous system: a report from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 99(6):2030–2037
Sklar CA, Mertens AC, Mitby P et al (2002) Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 87(7):3136–3141
Ergun-Longmire B, Mertens AC, Mitby P et al (2006) Growth hormone treatment and risk of second neoplasms in the childhood cancer survivor. J Clin Endocrinol Metab 91(9):3494–3498
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2016_2306_MOESM1_ESM.docx
Supplemental Figure 1: Diagram showing the flow of patients across the time of the study. Abbreviation: Y, yes; N, no; CSI, craniospinal irradiation; GHRT, Growth hormone replacement therapy; PNET, primitive neuroectodermal tumor. (DOCX 33 KB)
11060_2016_2306_MOESM2_ESM.docx
Supplemental Figure 2: Risk of secondary malignancies by treatment protocol and decade of primary diagnosis. Number of children treated on different time period (1980-1989: n=3; 1990-1999: n=9; 2000-2009: n=48; 2010-2013: n=5). (DOCX 2942 KB)
Rights and permissions
About this article
Cite this article
Indini, A., Schiavello, E., Biassoni, V. et al. Long-term safety of growth hormone replacement therapy after childhood medulloblastoma and PNET: it is time to set aside old concerns. J Neurooncol 131, 349–357 (2017). https://doi.org/10.1007/s11060-016-2306-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2306-7