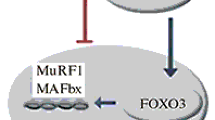
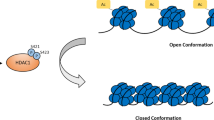
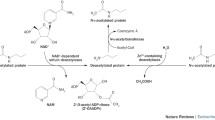
Two types of enzymes control the reversibility of the acetylation of amino acid lysine residues in histone molecules in the posttranslational modifications of proteins – histone acetyltransferases (HAT) and histone deacetylases (HDAC). HAT enzymes catalyze the transfer of an acetyl group to the amino acid lysine in the N-terminal parts of histones H2A, H2B, H3, and H4. HDAC enzymes are members of the hydrolases family and play an important role in signal transmission by deacetylating histones, inducing changes in gene expression in skeletal muscle in different functional states in humans. Of the 18 histone deacetylases, class IIa includes four enzymes (HDAC 4, 5, 7, and 9), which are tissue-specific and are involved in regulating metabolism in skeletal and cardiac muscles. Class IIa enzymes react with specific transcription factors to form multicomponent protein complexes, nuclear-cytoplasmic transfer of which in muscle cells influences the expression of regulatory and metabolic genes. Studies in recent years have provided evidence of the important role of this group of histone deacetylases in controlling intracellular metabolism and point to the need for studies of the molecular mechanisms involved in gene expression in skeletal muscles.
Similar content being viewed by others
References
I. V. Astratenkova and V. A. Rogozkin, “Involvement of AMP-dependent protein kinase in the regulation of metabolism in skeletal muscle,” Ros. Fiziol. Zh., 99, No. 6, 657–673 (2013).
I. V. Astratenkova and V. A. Rogozkin, “Molecular mechanisms of skeletal muscle hypertrophy,” Ros. Fiziol. Zh., 100, No. 6, 649–669 (2014).
I. V. Astratenkova and V. A. Rogozkin, “Signal pathways involved in regulating protein metabolism in skeletal muscle,” Ros. Fiziol. Zh., 102, No. 7, 753–772 (2016).
N. D. Gol’berg, A. M. Druzhevskaya, V. A. Rogozkin, and I. I. Akhmetov, “The role of mTOR in the regulation of metabolism in skeletal muscle,” Fiziol. Cheloveka, 40, No. 5, 123–132 (2014).
A. M. Druzhevskaya, I. I. Akhmetov, and V. A. Rogozkin, “Involvement of Akt in the regulation of metabolism in skeletal muscle,” Ros. Fiziol. Zh., 99, No. 4, 518–527 (2013).
V. Bulusu, S. Tumanov, E. Michalopoulou, et al., “Acetate recapturing by nuclear acetyl-CoA synthetase 2 prevents loss of histone acetylation during oxygen and serum limitation,” Cell Rep., 18, No. 2, 647–658 (2017).
B. M. Dancy and P. A. Cole, “Protein lysine acetylation by p300/CBP,” Chem. Rev., 115, No. 6, 24–52 (2015).
S. Das, F. Morvan, B. Jourde, et al., “ATP citrate lyase improves mitochondrial function in skeletal muscle,” Cell Metab., 21, No. 6, 868–876 (2015).
E. Di Giorgio and C. Brancolini, “Regulation of class IIa HDAC activities: it is not only matter of subcellular localization,” Epigenomics, 8, No. 2, 251–269 (2016).
J. Fan, K. A. Krautkramer, J. L. Feldman, and J. M. Denu, “Metabolic regulation of histone post-translational modifications,” ASC Chem. Biol., 10, No. 1, 95–108 (2015).
L. Galluzzi, J. M. Bravo-San Pedro, I. Vitale, S. A. Aaronson, et al., “Essential versus accessory aspects of cell death: recommendations of the NCCD 2015,” Cell Death Differ., 22, No. 1, 58–73 (2015).
V. Gaur, T. Connor, A. Sanigorski, et al., “Disruption of the Class IIa HDAC corepressor complex increases energy expenditure and lipid oxidation,” Cell Rep., 16, No. 11, 2802–2810 (2016).
A. J. Guise, R. A. Mathias, E. A. Rowland, et al., “Probing phosphorylation-dependent protein interactions within functional domains of histone deacetylase 5 (HDAC5),” Proteomics, 14, No. 19, 2156–2166 (2014).
K. F. Howlett and S. L. McGee, “Epigenetic regulation of skeletal muscle metabolism,” Clin. Sci. (Lond.), 130, No. 13, 1051–1063 (2016).
G. M. Hudson, P. J. Watson, L. Fairall, et al., “Insights into the recruitment of class IIa Histone Deacetylases (HDACs) to the SMRT/NCoR transcriptional complex,” J. Biol. Chem., 290, No. 29, 18237–18244 (2015).
W. G. Kaelin and S. L. McKnight, “Influence of metabolism on epigenetics and disease,” Cell, 153, No. 1, 56–69 (2013).
J. V. Lee, S. A. Shah, and K. E. Wellen, “Obesity, cancer and acetyl-CoA metabolism,” Drug Discov. Today Dis. Mech., 10, No. 1–2, e55–e61 (2013).
N. Liu, B. R. Nelson, S. Bezprozvannaya, et al., “Requirement of MEF2A, C, D for skeletal muscle regeneration,” Proc. Natl. Acad. Sci. USA, 111, No. 11, 4109–4114 (2014).
P. Madiraju, S. V. Pande, M. Prentki, and S. R. Madiraju, “Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation,” Epigenomics, 4, No. 6, 399–403 (2009).
G. Mansueto, A. Armani, C. Viscomi, et al., “Transcription factor EB controls metabolic flexibility during exercise,” Cell Metab., 25, No. 1, 182–196 (2017).
R. Marmorstein and M. M. Zhou, “Writers and readers of histone acetylation: structure, mechanism, and inhibition,” Cold Spring Harb. Perspect. Biol., 6, No. 7, a018762 (2014).
C. E. McCullough and R. Marmorstein, “Molecular basis for histone acetyltransferase regulation by binding partners, associated domains and autoacetylation,” ACS Chem., 11, No. 3, 632–642 (2016).
S. L. McGee, E. Fairlie, A. P. Garnham, and M. Hargreaves, “Exercise-induced histone modifications inhuman skeletal muscle,” J. Physiol., 587, No. 17, 5951–5958 (2009).
S. L. McGee and M. Hargreaves, “Histone modifications and skeletal muscle metabolic gene expression,” Clin. Exp. Pharmacol. Physiol., 37, No. 3, 392–396 (2010).
S. L. McGee, C. Swinton, S. Morrison, et al., “Compensatory regulation of HDAC5 in muscle maintains metabolic adaptive responses and metabolism in response to energetic stress,” FASEB J., 28, No. 8, 3384–3395 (2014).
D. L. Medina, S. Di Paola, I. Peluso, et al., “Lysosomal calcium signaling regulates autophagy via calcineurin and TFEB,” Nat. Cell Biol., 17, No. 3, 288–299 (2015).
I. Moretti, S. Ciciliot, K. A. Dyar, et al., “MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity,” Nat. Commun., 7, 12397 (2016), doi: 10.1038.2016.
M. Parra, “Class IIa HDACs - new insights into their functions in physiology and pathology,” FEBS J., 282, No. 9, 1736–1744 (2015).
F. Pietrola, L. Galluzzi, J. M. Bravo-San Pedro, et al., “Acetyl Coenzyme A: a central metabolic and second messenger,” Cell Metab., 21, No. 6, 805–821 (2015).
J. R. Pon and M. A. Marra, “MEf2 transcription factors: developmental regulators and emerging cancer genes,” Oncotarget, 7, No. 3, 2297–2312 (2016).
M. J. Potthoff, H. Wu, M. A. Arnold, et al., “Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers,” J. Clin. Invest., 117, No. 9, 2459–2467 (2007).
Z. T. Schug, B. Peck, D. T. Jones, et al., “Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress,” Cancer Cell, 27, No. 1, 57–71 (2015).
L. Shi and P. T. Benjamin, “Acetyl-CoA and the regulation of metabolism: mechanisms and consequences,” Curr. Opin. Cell Biol., 33, No. 1, 125–131 (2015).
L. Shi and B. P. Tu, “Protein acetylation as a means to regulate protein function in tune with metabolic state,” Biochem. Soc. Trans., 42, No. 4, 1037–1042 (2014).
K. K. Starheim, K. Gevaert, and T. Arnesen, “Protein N-terminal acetyltransferases: when the start matters,” Trends Biochem. Sci., 37, No. 4, 152–161 (2012).
X. Su, K. E. Wellen, and J. D. Rabinowitz, “Metabolic control of methylation and acetylation,” Curr. Opin. Chem. Biol., 30, 52–60 (2016).
G. Sutendra, A. Kinnaird, P. Dromparis, et al., “A nuclear pyruvate dehydrogenase complex is important for generation of acetyl-CoA and histone acetylation,” Cell, 158, No. 1, 84–97 (2014).
F. Vahid, H. Zand, E. Nosrat-Mirshekarlou, et al., “The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: a review,” Gene, 562, No. 1, 8–15 (2015).
M. E. Walsh, and H. Van Remmen, “Emerging roles for histone deacetylases in age-related muscle atrophy,” Nutr. Healthy Aging, 4, No. 1, 17–30 (2016).
Z. Wang, G. Qin, and T. C. Zhao, “Histone deacetylase 4 (HDAC4): mechanism regulations and biological function,” Epigenomics, 6, No. 1, 139–150 (2014).
C. Yang, B. Ko, C. T. Hensley, et al., “Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport,” Mol. Cell., 56, No. 3, 414–424 (2014).
S. Zhao, A. Torres, R. A. Henry, et al., “ATP-citrate lyase controls a glucose-to-acetate metabolic switch,” Cell Rep., 17, No. 4, 1037–1052 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 103, No. 6, pp. 593–605, June, 2017.
Rights and permissions
About this article
Cite this article
Astratenkova, I.V., Rogozkin, V.A. The Role of Acetylation/Deacetylation of Histones and Transcription Factors in Regulating Metabolism in Skeletal Muscles. Neurosci Behav Physi 49, 281–288 (2019). https://doi.org/10.1007/s11055-019-00730-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00730-2