Abstract
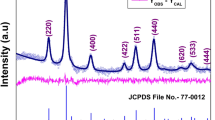
Octahedral magnetite (Fe3O4) nanoparticles (NPs) with dimensions ranging from 20 to 110 nm are prepared via the decomposition of iron oleate complex in the presence of cetyltrimethylammonium bromide (CTAB) and various organic solvents. It is shown that the addition of an optimal amount of CTAB prompts the growth of equi-dimensional octahedral NPs with dominant {111} facets. Moreover, it is shown that the size of the octahedral NPs can be controlled by adjusting the relative amounts of oleic acid and CTAB and choosing an appropriate high-boiling point organic solvent. The X-ray diffraction analysis results reveal that the compositions of the as-synthesized NPs are solvent-dependent and contain different amounts of iron metal, wüstite and magnetite phases. The as-synthesized NPs are oxidized to pure magnetite via an air treatment and the room-temperature magnetic properties of the resulting products are then characterized by means of a superconducting quantum interference device. The results indicate that the superparamagnetic limit for the current magnetite NPs is equal to approximately 33.3 nm at a temperature of 300 K.
Graphical Abstract







Similar content being viewed by others
References
Asokan S, Krueger KM, Colvin VL, Wong MS (2007) Shape-controlled synthesis of CdSe tetrapods using cationic surfactant ligand. Small 3(7):1164–1169
Chen CJ, Lai HY, Lin CC, Wang JS, Chiang RK (2009) Preparation of monodisperse iron oxide nanoparticles via the synthesis and decomposition of iron fatty acid complexes. Nanoscale Res Lett 4:1343–1350
Chen CJ, Chiang RK, Jeng YR (2011) Crystallization and magnetic properties of 3D micrometer-scale simple-cubic maghemite superlattices. J Phys Chem C 115:18142–18148
Cheon J, Kang NJ, Lee SM, Lee JH, Yoon JH, Oh SJ (2004) Shape evolution of single-crystalline iron oxide nanocrystals. J Am Chem Soc 126(7):1950–1951
Daou TJ, Pourroy G, Bégin-Colin S, Grenèche JM, Ulhaq-Bouillet C, Legaré P, Bernhardt P, Leuvrey C, Rogez G (2006) Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem Mater 18(18):4399–4404
Faivre D, Schüler D (2008) Magnetotactic bacteria and magnetosomes. Chem Rev 108(11):4875–4898
Hai HT, Kura H, Takahashi M, Ogawa T (2010) Facile synthesis of Fe3O4 nanoparticles by reduction phase transformation from γ-Fe2O3 nanoparticles in organic solvent. J Colloid Interface Sci 341:194–199
Hergt R, Dutz S, Müller R, Zeisberger M (2006) Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. J Phys Condens Matter 18:S2919–S2934
Jun YW, Choi JS, Cheon J (2006) Shape control of semiconductor and metal oxide nanocrystals through nonhydrolytic colloidal routes. Angew Chem Int Ed 45:3414–3439
Jun YW, Seo JW, Cheon J (2007) Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical science. Acc Chem Res 41(2):179–189
Kim D, Lee N, Park M, Kim BH, An K, Hyeon T (2009) Synthesis of uniform ferromagnetic magnetite nanocubes. J Am Chem Soc 131(2):454–455
Kittel C (1946) Theory of the structure of ferromagnetic domains in films and small particles. Phys Rev 70(11):965–971
Kovalenko MV, Bodnarchuk MI, Lechner RT, Hesser G, Schäffler F, Heiss W (2007) Fatty acid salts as stabilizers in size- and shape-controlled nanocrystal synthesis: the case of inverse spinel iron oxide. J Am Chem Soc 129(20):6352–6353
Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterization, and biological applications. Chem Rev 108(6):2064–2110
Lee SM, Cho SN, Cheon J (2003) Anisotropic shape control of colloidal inorganic nanocrystals. Adv Mater 15(5):441–444
Leslie-Pelecky DL, Rieke RD (1996) Magnetic properties of nanostructured materials. Chem Mater 8(8):1770–1783
Li L, Yang Y, Ding J, Xue J (2010) Synthesis of magnetite nanooctahedra and their magnetic field-induced two-/three-dimensional superstructure. J Chem Mater 22(10):3183–3191
Lim B, Jiang M, Tao J, Camargo PHC, Zhu Y, Xia Y (2009) Shape-controlled synthesis of Pd nanocrystals in aqueous solutions. Adv Funct Mater 19:189–200
Liu P, Cai W, Zeng H (2008) Fabrication and size-dependent optical properties of FeO nanoparticles induced by laser ablation in a liquid medium. J Phys Chem C 112(9):3261–3266
Lu W, Liu Q, Sun Z, He J, Ezeolu C, Fang J (2008) Super crystal structures of octahedral c-In2O3 nanocrystals. J Am Chem Soc 130(22):6983–6991
Manna L, Milliron DJ, Meisel A, Scher EC (2003) Controlled growth of tetrapod-branched inorganic nanocrystals. Nat Mater 2:382–385
Muxworthy AR, Williams W (2009) Critical superparamagnetic/single-domain grain sizes in interacting magnetic particles: implications for magnetosome crystals. J R Soc Interface 6:1207–1212
Nie Z, Petukhova A, Kumacheva E (2010) Properties and emerging applications of self-assembled structure made from inorganic nanoparticles. Nat Nanotechnol 5:15–25
Niu W, Zheng S, Wang D, Liu X, Li H, Han S, Chen J, Tang Z, Xu G (2009) Selective synthesis of single-crystalline rhombic dodecahedral, octahedral, and cubic gold nanocrystals. J Am Chem Soc 13(2):697–703
O’Handley RC (2000) Modern magnetic materials: principles and applications. Wiley, New York
Ould-Ely T, Prieto-Centurion D, Kumar A, Guo W, Knowles WV, Asokan S, Wong MS, Rusakova I, Lüttge A, Whitmire KH (2006) Manganese(II) oxide nanohexapods: insight into controlling the form of nanocrystals. Chem Mater 18(7):1821–1829
Pileni MP (2003) The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat Mater 2:145–150
Pileni MP (2007) Control of the size and shape of inorganic nanocrystals at various scales from nano to macrodomains. J Phys Chem C 111(26):9019–9038
Redl FX, Black CT, Papaefthymiou GC, Sandstrom RL, Yin M, Zeng H, Murray CB, O’Brien SP (2004) Magnetic, electronic, and structural characterization of nonstoichiometric iron oxide at the nanoscale. J Am Chem Soc 126(44):14583–14599
Salazar-Alvarez G, Qin J, Šepelák V, Bergmann I, Vasilakaki M, Trohidou KN, Ardisson JD, Macedo WAA, Mikhaylova M, Muhammed M, Baró MD, Nogués J (2008) Cubic versus spherical magnetic nanoparticles: the role of surface anisotropy. J Am Chem Soc 130(40):13234–13239
Shavel A, Rodríguez-González B, Pacifico J, Spasova M, Farle M, Liz-Marzán LM (2009) Shape control in iron oxide nanocrystal synthesis, induced by trioctylammonium ions. Chem Mater 21(7):1326–1332
Siegfried MJ, Choi KS (2006) Elucidating the effects of additives on the growth and stability of Cu2O surface via shape transformation of pre-grown crystals. J Am Chem Soc 128(32):10356–10357
Song Q, Ding Y, Wang ZL, Zhang ZJ (2006) Formation of orientation-ordered superlattices of magnetite magnetic nanocrystals from shape-segregated self-assemblies. J Phys Chem B 110(50):25547–25550
Sorenson CM (2001) Nanoscale materials in chemistry. Wiley, New York
Tang J, Myers M, Bosnick KA, Brus LE (2003) Magnetite Fe3O4 nanocrystals: spectroscopic observation of aqueous oxidation kinetics. J Phys Chem B 107(30):7501–7506
Tao AR, Habas S, Yang P (2008) Shape control of colloidal metal nanocrystals. Small 4(3):310–325
Teng X, Yang H (2004) Effects of surfactants and synthetic conditions on the sizes and self-assembly of monodisperse iron oxide nanoparticles. J Mater Chem 14:774–779
Tian N, Shou ZY, Sun SG, Ding Y, Wang ZL (2007) Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316:732–735
Yin Y, Alivisatos AP (2005) Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 437:664–670
Zhao L, Zhang H, Xing Y, Song S, Yu S, Shi W, Guo X, Yang J, Lei Y, Cao F (2008) Morphology-controlled synthesis of magnetites with nanoporous structures and excellent magnetic properties. Chem Mater 20(1):198–204
Acknowledgments
The authors gratefully acknowledge the financial support provided to this study by the National Science Council of the Republic of China, Taiwan, under Contract No. NSC 99-2113-M-269-001.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, CJ., Chiang, RK., Wang, JS. et al. Synthesis and magnetic properties of octahedral magnetite nanoparticles in 20–110 nm range. J Nanopart Res 15, 1845 (2013). https://doi.org/10.1007/s11051-013-1845-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1845-5