Abstract
Background
An increase in cancer stem cell (CSC) populations and their resistance to common treatments could be a result of c-Myc dysregulations in certain cancer cells. In the current study, we investigated anticancer effects of c-Myc decoy ODNs loaded-poly (methacrylic acid-co-diallyl dimethyl ammonium chloride) (PMA-DDA)-coated silica nanoparticles as carriers on cancer-like stem cells (NTERA-2).
Methods and results
The physicochemical characteristics of the synthesized nanocomposites (SiO2@PMA-DDA-DEC) were analyzed using FT-IR, DLS, and SEM techniques. UV–Vis spectrophotometer was applied to analyze the release pattern of decoy ODNs from the nanocomposite. Furthermore, uptake, cell viability, apoptosis, and cell cycle assays were used to investigate the anticancer effects of nanocomposites loaded with c-Myc decoy ODNs on NTERA-2 cancer cells. The results of physicochemical analytics demonstrated that SiO2@PMA-DDA-DEC nanocomposites were successfully synthesized. The prepared nanocomposites were taken up by NTERA-2 cells with high efficiency, and could effectively inhibit cell growth and increase apoptosis rate in the treated cells compared to the control group. Moreover, SiO2@PMA-DDA nanocomposites loaded with c-Myc decoy ODNs induced cell cycle arrest at the G0/G1 phase in the treated cells.
Conclusions
The conclusion drawn from this study is that c-Myc decoy ODN-loaded SiO2@PMA-DDA nanocomposites can effectively inhibit cell growth and induce apoptosis in NTERA-2 cancer cells. Moreover, given that a metal core is incorporated into this synthetic nanocomposite, it could potentially be used in conjunction with irradiation as part of a decoy-radiotherapy combinational therapy in future investigations.
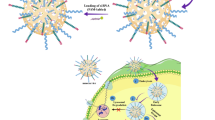
Graphical abstract










Similar content being viewed by others
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AgNPs:
-
Silver nanoparticles
- BSA:
-
Bovine serum albumin
- EDC:
-
1-(3-Dimethyl aminopropyl)-3-ethylcarbodiimide hydrochloride
- FT-IR:
-
Fourier transform infrared
- PI:
-
Propidium iodide
- UV–Vis:
-
Ultraviolet–visible
- FESEM:
-
Field emission scanning electron microscopy
- DLS:
-
Dynamic light scattering
- PDI:
-
Polydispersity Index
- PMA-DDA:
-
Poly (methacrylic acid-co-diallyl dimethyl ammonium chloride)
- CSC:
-
Cancer stem cell
- TFD:
-
Transcription factor decoy
- ODN:
-
Oligodeoxynucleotide
- DADMAC:
-
Diallyl dimethyl ammonium chloride
- PBS:
-
Phosphate-buffered saline
- DMSO:
-
Dimethyl sulfoxide
- SEM:
-
Scanning electron microscopy
- EDTA:
-
Ethylenediamine tetra-acetic acid
- DEC:
-
Decoy
- SCR:
-
Scramble
- SiO2 :
-
Silicon dioxide
References
Ginsburg O et al (2017) The global burden of women’s cancers: a grand challenge in global health. Lancet 389(10071):847–860
Zhou B et al (2017) Early detection of pancreatic cancer: where are we now and where are we going? Int J Cancer 141(2):231–241
Peiris-Pagès M et al (2016) Cancer stem cell metabolism. Breast Cancer Res 18(1):1–10
Clarke MF et al (2006) Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Can Res 66(19):9339–9344
Thomassen I et al (2013) Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis Colon Rectum 56(12):1373–1380
Ali Azouaou S et al (2015) Selective ROS-dependent p53-associated anticancer effects of the hypoxoside derivative rooperol on human teratocarcinomal cancer stem-like cells. Invest New Drugs 33(1):64–74
Schmidtova S et al (2019) Disulfiram overcomes cisplatin resistance in human embryonal carcinoma cells. Cancers 11(9):1224
Knight SR et al (2021) Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet 397(10272):387–397
Roy A, Li SD (2016) Modifying the tumor microenvironment using nanoparticle therapeutics. Wiley Interdiscip Rev 8(6):891–908
Han L et al (2013) Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharm Sin B 3(2):65–75
Brivanlou AH, Darnell JE Jr (2002) Signal transduction and the control of gene expression. Science 295(5556):813–818
Dreesen O, Brivanlou AH (2007) Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev 3(1):7–17
Palomero T et al (2006) NOTCH1 directly regulates c-Myc and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci 103(48):18261–18266
Gandarillas A, Watt FM (1997) c-Myc promotes differentiation of human epidermal stem cells. Genes Dev 11(21):2869–2882
Iritani BM, Eisenman RN (1999) c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci 96(23):13180–13185
Johnston LA et al (1999) Drosophila Myc regulates cellular growth during development. Cell 98(6):779–790
Schmidt EV (1999) The role of c-Myc in cellular growth control. Oncogene 18(19):2988–2996
Schuhmacher M et al (1999) Control of cell growth by c-Myc in the absence of cell division. Curr Biol 9(21):1255–1258
Packham G, Cleveland JL (1995) c-Myc and apoptosis. Biochim Biophys Acta 1242(1):11–28
Hoffman B, Liebermann DA (1998) The proto-oncogene c-Myc and apoptosis. Oncogene 17(25):3351–3357
Dang CV (1999) c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol 19(1):1–11
Dang CV et al (1999) Function of the c-Myc oncogenic transcription factor. Exp Cell Res 253(1):63–77
Elend M, Eilers M (1999) Cell growth: downstream of Myc–to grow or to cycle? Curr Biol 9(24):R936–R938
Prendergast GC (1999) Mechanisms of apoptosis by c-Myc. Oncogene 18(19):2967–2987
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Miller DM et al (2012) c-Myc and cancer metabolism. Clin Cancer Res 18(20):5546–5553
Mann MJ, Dzau VJ (2000) Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Investig 106(9):1071–1075
Hecker M, Wagner AH (2017) Transcription factor decoy technology: a therapeutic update. Biochem Pharmacol 144:29–34
Rahmati M et al (2020) Suppressing the metastatic properties of the breast cancer cells using STAT3 decoy oligodeoxynucleotides: a promising approach for eradication of cancer cells by differentiation therapy. J Cell Physiol 235(6):5429–5444
Johari B et al (2020) Evaluation of STAT3 decoy oligodeoxynucleotides’ synergistic effects on radiation and/or chemotherapy in metastatic breast cancer cell line. Cell Biol Int 44(12):2499–2511
Bigdelou Z et al (2020) Role of Oct4–Sox2 complex decoy oligodeoxynucleotides strategy on reverse epithelial to mesenchymal transition (EMT) induction in HT29-ShE encompassing enriched cancer stem-like cells. Mol Biol Rep 47(3):1859–1869
Johari B et al (2017) Myc decoy oligodeoxynucleotide inhibits growth and modulates differentiation of mouse embryonic stem cells as a model of cancer stem cells. Anti-Cancer Agents Med Chem 17(13):1786–1795
Ashby BS (1966) pH studies in human malignant tumours. Lancet 288(7458):312–315
Engin K et al (1995) Extracellular pH distribution in human tumours. Int J Hyperth 11(2):211–216
Naeslund J, Swenson K-E (1953) Investigations on the pH of malignant tumours in mice and humans after the administration of glucose. Acta Obstet Gynecol Scand 32(3):359–367
Hao G, Xu ZP, Li L (2018) Manipulating extracellular tumour pH: an effective target for cancer therapy. RSC Adv 8(39):22182–22192
Li D, He Q, Li J (2009) Smart core/shell nanocomposites: intelligent polymers modified gold nanoparticles. Adv Coll Interface Sci 149(1–2):28–38
Kalele S et al (2006) Nanoshell particles: synthesis, properties and applications. Curr Sci 91:1038–1052
West JL, Halas NJ (2003) Engineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeutics. Annu Rev Biomed Eng 5(1):285–292
Liu F et al (2015) Positively charged loose nanofiltration membrane grafted by diallyl dimethyl ammonium chloride (DADMAC) via UV for salt and dye removal. React Funct Polym 86:191–198
Farhad-Gholami N et al (2023) Sustained doxorubicin delivery system to breast tumor cancer cell based on a novel cationic molecularly imprinted polymer. Int J Polym Mater Polym Biomater 72(4):335–344
Mohammadzade H et al (2023) Molecular imprinting of miR-559 on a peptide-immobilized poly L-DOPA/silica core–shell and in vitro investigating its effects on HER2-positive breast cancer cells. Drug Deliv Transl Res 13:1–16
Johari B, Zargan J (2017) Simultaneous targeted inhibition of Sox2-Oct4 transcription factors using decoy oligodeoxynucleotides to repress stemness properties in mouse embryonic stem cells. Cell Biol Int 41(12):1335–1344
Johari B et al (2023) Combinational therapy with Myc decoy oligodeoxynucleotides encapsulated in nanocarrier and X-irradiation on breast cancer cells. Oncol Res 32(2):309
Ghorbani R et al (2023) Targeted anti-tumor synergistic effects of Myc decoy oligodeoxynucleotides-loaded selenium nanostructure combined with chemoradiotherapy on LNCaP prostate cancer cells. Oncol Res 32(1):101
Gharbavi M et al (2020) Cholesterol-conjugated bovine serum albumin nanoparticles as a tamoxifen tumor-targeted delivery system. Cell Biol Int 44(12):2485–2498
Watermann A, Brieger J (2017) Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 7(7):189
Li T et al (2019) Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater 89:1–13
Feng Y et al (2016) The application of mesoporous silica nanoparticle family in cancer theranostics. Coord Chem Rev 319:86–109
Mirzababaei M et al (2022) Graphene quantum dots coated cationic polymer for targeted drug delivery and imaging of breast cancer. J Polym Res 30(7):268
Farhad-Gholami N et al (2021) Sustained doxorubicin delivery system to breast tumor cancer cell based on a novel cationic molecularly imprinted polymer. Int J Polym Mater Polym Biomater 72:1–10
Ghorbani R et al (2024) Targeted anti-tumor synergistic effects of Myc decoy oligodeoxynucleotides-loaded selenium nanostructure combined with chemoradiotherapy on LNCaP prostate cancer cells. Oncol Res 32(1):101–125
Bretones G, Delgado MD, León J (2015) Myc and cell cycle control. Biochim Biophys Acta 1849(5):506–516
Fan Y et al (2020) Radiosensitizing effects of c-Myc gene knockdown-induced G2/M phase arrest by intrinsic stimuli via the mitochondrial signaling pathway. Oncol Rep 44(6):2669–2677
Sabit H et al (2021) Knockdown of c-MYC controls the proliferation of oral squamous cell carcinoma cells in vitro via dynamic regulation of key apoptotic marker genes. Int J Mol Cell Med 10(1):45
Acknowledgements
The Medical Biotechnology Department staff of Zanjan University of Medical Sciences have been praised for their assistance by the authors.
Funding
The grant was received by Behrooz Johari from Zanjan University of Medical Sciences (Grant Number: A-12-1244-24 & Ethical Code: IR.ZUMS.BLC.1402.014).
Author information
Authors and Affiliations
Contributions
Roghayeh Ghorbani: Methodology, writing the original draft. Mahmoud Gharbavi: Methodology, review and editing. Benyamin Keshavarz: Methodology. Hamid Madanchi: Methodology, conceptualization, software, writing the original draft, and editing. Behrooz Johari: Supervision, project administration, conceptualization, writing the original draft, review, and editing, and final approval.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare no financial or commercial conflict of interest that could negatively influence the study.
Ethical approval
There was no human participant and consent was not required and no human or animal was involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghorbani, R., Gharbavi, M., Keshavarz, B. et al. Targeting c-Myc with decoy oligodeoxynucleotide-loaded polycationic nanoparticles inhibits cell growth and induces apoptosis in cancer stem-like cells (NTERA-2). Mol Biol Rep 51, 623 (2024). https://doi.org/10.1007/s11033-024-09559-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09559-6