Abstract
Background
The non-alcoholic fatty liver disease (NAFLD) is prevalent in as many as 25% of adults who are afflicted with metabolic syndrome. Oxidative stress plays a significant role in the pathophysiology of hepatic and renal injury associated with NAFLD. Therefore, probiotics such as Lactobacillus casei (LBC) and the microalga Chlorella vulgaris (CV) may be beneficial in alleviating kidney injury related to NAFLD.
Materials and methods
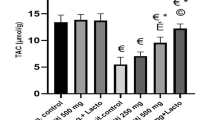
This animal study utilized 30 C57BL/6 mice, which were evenly distributed into five groups: the control group, the NAFLD group, the NAFLD + CV group, the NAFLD + LBC group, and the NAFLD + CV + LBC group. A high-fat diet (HFD) was administered to induce NAFLD for six weeks. The treatments with CV and LBC were continued for an additional 35 days. Biochemical parameters, total antioxidant capacity (TAC), and the expression of kidney damage marker genes (KIM 1 and NGAL) in serum and kidney tissue were determined, respectively. A stereological analysis was conducted to observe the structural changes in kidney tissues.
Results
A liver histopathological examination confirmed the successful induction of NAFLD. Biochemical investigations revealed that the NAFLD group exhibited increased ALT and AST levels, significantly reduced in the therapy groups (p < 0.001). The gene expression levels of KIM-1 and NGAL were elevated in NAFLD but were significantly reduced by CV and LBC therapies (p < 0.001). Stereological examinations revealed reduced kidney size, volume, and tissue composition in the NAFLD group, with significant improvements observed in the treated groups (p < 0.001).
Conclusion
This study highlights the potential therapeutic efficacy of C. vulgaris and L. casei in mitigating kidney damage caused by NAFLD. These findings provide valuable insights for developing novel treatment approaches for managing NAFLD and its associated complications.






Similar content being viewed by others
Data availability
This article contains all data created and examined throughout this investigation. The corresponding author will provide datasets used or analyzed during the current work upon reasonable request.
Abbreviations
- ALT:
-
Alanine Aminotransferase
- AST:
-
Aspartate Aminotransferase
- BUN:
-
Blood Urea Nitrogen
- Chol:
-
Cholesterol
- CKD:
-
Chronic Kidney Disease
- CV:
-
Chlorella vulgaris
- CVD:
-
Cardio vascular Diseases
- DCT:
-
Distal convoluted tubule
- FOXO1:
-
Forkhead box protein O1
- FRAP:
-
Ferric reducing Antioxidant Power
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- H&E:
-
Hematoxylin and Eosin
- HFD:
-
High-fat diet
- KIM-1:
-
Kidney Injury Molecule-1
- LBC:
-
Lactobacillus casei
- MAFLD:
-
Metabolic-Associated Fatty Liver disease
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- OS:
-
Oxidative stress
- p-AMPK:
-
Phosphorylated AMP-activated protein kinase
- PCT:
-
Proximal convoluted tubule
- ROS:
-
Reactive Oxygen Species
- RT-PCR:
-
Real-Time Polymerase Chain Reaction
- T2D:
-
Type 2 diabetes
- TAC:
-
Total Antioxidant Capacity
- TG:
-
Triglyceride
- TPTZ:
-
2,4,6 tripyridyl-s-triazine
References
Choi J, Joe H, Oh J-E, Cho Y-J, Shin H-S, Heo NH (2023) The correlation between NAFLD and serum uric acid to serum creatinine ratio. PLoS ONE 18:e0288666
Pouwels S, Sakran N, Graham Y et al (2022) Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disorders 22:1–9
Pai RK (2019) NAFLD histology: a critical review and comparison of scoring systems. Curr Hepatol Rep 18:473–481
Zheng X, Cao C, He Y, Wang X, Wu J, Hu H (2021) Association between nonalcoholic fatty liver disease and incident diabetes mellitus among Japanese: a retrospective cohort study using propensity score matching. Lipids Health Dis 20:59
Zhang M, Lin S, Wang M-f et al (2020) Association between NAFLD and risk of prevalent chronic kidney disease: why there is a difference between east and west? BMC Gastroenterol 20:1–7
Mantovani A, Lombardi R, Cattazzo F, Zusi C, Cappelli D, Dalbeni A (2022) MAFLD and CKD: an updated narrative review. Int J Mol Sci 23:7007–7007
Lonardo A, Mantovani A, Targher G, Baffy G (2022) Nonalcoholic fatty liver disease and chronic kidney disease: epidemiology, pathogenesis, and clinical and research implications. Int J Mol Sci 23:13320–13320
Hydes T, Buchanan R, Kennedy OJ, Fraser S, Parkes J, Roderick P (2020) Systematic review of the impact of non-alcoholic fatty liver disease on mortality and adverse clinical outcomes for individuals with chronic kidney disease. BMJ Open 10:e040970–e040970
Gonzalez A, Huerta-Salgado C, Orozco-Aguilar J et al (2020) Role of oxidative stress in hepatic and extrahepatic dysfunctions during nonalcoholic fatty liver Disease (NAFLD). Oxidative Med Cell Longev 2020:1617805–1617805
Delli Bovi AP, Marciano F, Mandato C, Siano MA, Savoia M, Vajro P (2021) Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front Med 8:165–165
Nielsen S, Schjoedt K, Astrup A et al (2009) Kidney injury molecule 1 (KIM1) and neutrophil gelatinase-associated lipocalin (NGAL) as markers of tubular kidney injury in type 1 diabetic patients
Abourady AA, Amin SM, Kasem SM, Abdelnaby HH (2020) Measurement of urinary kidney injury molecule-1 level as an early biomarker of renal impairment in overweight/obese children and adolescents with nonalcoholic fatty liver disease. J Am Sci 16
Chen L, Lv X, Kan M, Wang R, Wang H, Zang H (2022) Critical overview of hepatic factors that link non-alcoholic fatty liver disease and acute kidney Injury: physiology and therapeutic implications. Int J Mol Sci 23:12464
Francoz C, Nadim MK, Durand F (2016) Kidney biomarkers in cirrhosis. J Hepatol 65:809–824
David D, Eapen CE (2021) What are the current pharmacological therapies for nonalcoholic fatty liver disease? J Clin Experimental Hepatol 11:232–238
Shao Y, Chen S, Han L, Liu J (2023) Pharmacotherapies of NAFLD: updated opportunities based on metabolic intervention. Nutr Metabolism 20:30
Abdella A, Elbadawy MF, Irmak S, Alamri ES (2022) Hypolipidemic, antioxidant and Immunomodulatory effects of Lactobacillus casei ATCC 7469-Fermented wheat Bran and Spirulina maxima in rats Fed a High-Fat Diet. Fermentation 8:610–610
Lee NY, Shin MJ, Youn GS et al (2021) Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin Mol Hepatol 27:110–110
Machu L, Misurcova L, Vavra Ambrozova J et al (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20:1118–1133
Wan M, Li Q, Lei Q, Zhou D, Wang S (2022) Polyphenols and polysaccharides from morus alba l. fruit attenuate high-fat diet-induced metabolic syndrome modifying the gut microbiota and metabolite profile. Foods 11:1818–1818
Panahi Y, Ghamarchehreh ME, Beiraghdar F, Zare R, Jalalian HR, Sahebkar A (2012) Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Hepatogastroenterology 59:2099–2103
Mohseni R, Alavian SM, Sadeghabadi ZA, Heiat M (2021) Therapeutic effects of Chlorella vulgaris on carbon tetrachloride induced liver fibrosis by targeting Hippo signaling pathway and AMPK/FOXO1 axis. Mol Biol Rep 48:117–126
An H-J, Choi H-M, Park H-S et al (2006) Oral administration of hot water extracts of Chlorella vulgaris increases physical stamina in mice. Annals Nutr Metabolism 50:380–386
Zhang Z, Zhou H, Guan M et al (2021) Lactobacillus casei YRL577 combined with plant extracts reduce markers of non-alcoholic fatty liver disease in mice. Br J Nutr 125:1081–1091
Karp NA, Meehan TF, Morgan H et al (2015) Applying the ARRIVE guidelines to an in vivo database. PLoS Biol 13, e1002151
Cho J, Lee I, Kim D et al (2014) Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exerc Nutr Biochem 18:339
Zhang Z, Zhou H, Zhou X et al (2021) Lactobacillus casei YRL577 ameliorates markers of non-alcoholic fatty liver and alters expression of genes within the intestinal bile acid pathway. Br J Nutr 125:521–529
Lim CSH, Lim SL (2013) Ferric reducing capacity versus ferric reducing antioxidant power for measuring total antioxidant capacity. Lab Med 44:51–55
Scherle W (1970) A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26:57–60
Maleki MH, Nadimi E, Vakili O et al (2023) Bilirubin improves renal function by reversing the endoplasmic reticulum stress and inflammation in the kidneys of type 2 diabetic rats fed high-fat diet. Chemico-Biol Interact 378:110490
Roderburg C, Krieg S, Krieg A et al (2023) Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of chronic kidney disease (CKD). Eur J Med Res 28:153–153
Ebrahimi-Mameghani M, Aliashrafi S, Javadzadeh Y, AsghariJafarabadi M (2014) The effect of Chlorella vulgaris supplementation on liver enzymes, serum glucose and lipid profile in patients with non-alcoholic fatty liver disease. Health Promotion Perspect 4:107
Khani S, Hosseini M, Taheri H, Nourani MR M and, Imani Fooladi A (2012) A Probiotics as an alternative strategy for prevention and treatment of human diseases: a review. Inflammation & Allergy-Drug Targets (Formerly Current Drug Targets-Inflammation & Allergy)(Discontinued) 11, 79–89
Fagundes RAB, Soder TF, Grokoski KC, Benetti F, Mendes RH (2018) Probiotics in the treatment of chronic kidney disease: a systematic review. Brazilian J Nephrol 40:278–286
Elmeleh MI, Attia T, Elgendy H (2023) Protective effect of Chlorella vulgaris and Spirulina platensis against ThioacetamideInduced Hepatorenal toxicity in male rats. J Curr Veterinary Res 5:79–92
Regueiras A, Huguet Á, Conde T et al (2021) Potential anti-obesity, anti-steatosis, and anti-inflammatory properties of extracts from the microalgae Chlorella vulgaris and Chlorococcum amblystomatis under different growth conditions. Mar Drugs 20:9
Andy SY, Keeffe EB (2003) Elevated AST or ALT to nonalcoholic fatty liver disease. accurate predictor of disease prevalence?
Imamura Y, Mawatari S, Oda K et al (2021) Changes in body composition and low blood urea nitrogen level related to an increase in the prevalence of fatty liver over 20 years: a cross-sectional study. Hepatol Res 51:570–579
Gonzalez A, Huerta-Salgado C, Orozco-Aguilar J et al (2020) Role of oxidative stress in hepatic and extrahepatic dysfunctions during nonalcoholic fatty liver disease (NAFLD). Oxidative Medicine and Cellular Longevity 2020
Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clement K (2020) Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology 158:1881–1898
Karlsson C, Lindell K, Ottosson M, Sjöström L, Carlsson Br, Carlsson LMS (1998) Human adipose tissue expresses angiotensinogen and enzymes required for its Conversion to Angiotensin II1. J Clin Endocrinol Metabolism 83:3925–3929
Yang M, Geng C-A, Liu X, Guan M (2021) Lipid disorders in NAFLD and chronic kidney disease. Biomedicines 9:1405–1405
Yang M, Geng C-A, Liu X, Guan M (2021) Lipid disorders in NAFLD and chronic kidney disease. Biomedicines 9:1405
Zhou D, Pan Q, Shen F et al (2017) Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 7:1529
Wagnerberger S, Spruss A, Kanuri G et al (2013) Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. J Nutr Biochem 24:531–538
Song H, Han W, Yan F, Xu D, Chu Q, Zheng X (2016) Dietary Phaseolus vulgaris extract alleviated diet-induced obesity, insulin resistance and hepatic steatosis and alters gut microbiota composition in mice. J Funct Foods 20:236–244
Wan X, Li T, Liu D et al (2018) Effect of marine microalga Chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar Drugs 16:498
Lin L, Tan W, Pan X, Tian E, Wu Z, Yang J (2022) Metabolic syndrome-related kidney injury: a review and update. Front Endocrinol 13:904001–904001
Wanchai K, Yasom S, Tunapong W et al (2018) Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin Sci 132:1545–1563
Zhang W, Miikeda A, Zuckerman J et al (2021) Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Sci Rep 11:518–518
Zhu H, Cao C, Wu Z et al (2021) The probiotic L. Casei Zhang slows the progression of acute and chronic kidney disease. Cell Metabol 33:1926–1942
Lee HS, Park HJ, Kim MK (2008) Effect of Chlorella vulgaris on lipid metabolism in Wistar rats fed high fat diet. Nutr Res Pract 2:204–210
Mounir M, Ibijbijen A, Farih K, Rabetafika HN, Razafindralambo HL (2022) Synbiotics and their antioxidant properties, mechanisms, and benefits on human and animal health: a narrative review. Biomolecules 12:1443
Li Q, Zheng T, Ding H et al (2023) Exploring the benefits of Probiotics in Gut inflammation and Diarrhea—from an antioxidant perspective. Antioxidants 12:1342
Kleniewska P, Hoffmann A, Pniewska E, Pawliczak R (2016) The influence of probiotic Lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxidative medicine and cellular longevity 2016
Keshavarz Azizi Raftar S, Ashrafian F, Yadegar A et al (2021) The protective effects of live and pasteurized Akkermansia muciniphila and its extracellular vesicles against HFD/CCl4-induced liver injury. Microbiol Spectr 9:e00484–e00421
Altunkaynak ME, Özbek E, Altunkaynak BZ, Can İ, Unal D, Unal B (2008) The effects of high-fat diet on the renal structure and morphometric parametric of kidneys in rats. J Anat 212:845–852
Armitage JA, Lakasing L, Taylor PD et al (2005) Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol 565:171–184
Schulz C-A, Engström G, Nilsson J et al (2020) Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol Dialysis Transplantation 35:265–273
Allegretti AS, Parada XV, Endres P et al (2021) Urinary NGAL as a diagnostic and prognostic marker for acute kidney injury in cirrhosis: a prospective study. Clinical and translational gastroenterology 12
Acknowledgements
The present article was extracted from the thesis written by Haniyeh Keyghobadi has been submitted in Department of Biology, Zarghan Branch, Islamic Azad University, Zarghan, Iran.
Funding
Public, private nor nonprofit funding organizations did not provide any specific grants for this research.
Author information
Authors and Affiliations
Contributions
All experiments, statistical analysis, and figure preparation were conducted by H.K (Haniyeh Keyghobadi), H.B (Hadis bozorgpoursavadjani), N.M (Nazanin Mohammadipoor) and F.K (Farhad Koohpeyma). The initial draft of manuscript was written by M.N (Marzieh Nemati), F.D (Farshad Dehghani), and GH.K (Gholamhossein Keighobadi). All tests were set up and second draft of manuscript was written by F.k, and SD (Sanaz Dastghaib). A final manuscript proof was completed by SD who also contributed more funds to the project.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Research Ethics Committee of the Islamic Azad University-Arsanjan Branch (Fars Province) has ethically confirmed the study (Ethics Code: IR.IAU.A.REC.1402.065).
Consent for publication
Not applicable.
Conflict of interest
Authors affirm that they don’t have competitive interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keyghobadi, H., bozorgpoursavadjani, H., Koohpeyma, F. et al. Therapeutic potential of Lactobacillus casei and Chlorella vulgaris in high-fat diet-induced non-alcoholic fatty liver disease (NAFLD)-associated kidney damages: a stereological study. Mol Biol Rep 51, 613 (2024). https://doi.org/10.1007/s11033-024-09542-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09542-1