Abstract
Objective
The prevalence of allergic rhinitis is high, making it a relatively common chronic condition. Countless patients suffer from seasonal Allergic rhinitis (AR). The objective of this investigation is to examine the potential involvement of common pollen allergens in seasonal allergic rhinitis, and study the proposed mechanism of Toll-like receptor 4 (TLR4)/Myeloid differentiation primary response gene 88 (MyD88) signaling pathway in the induction of AR.
Method
A mouse AR model (sensitized group) was constructed with pollen extracts and ovalbumin (OVA) of Artemisia annua (An), Artemisia argyi (Ar) and Artemisia Sieversiana (Si), and thereafter, AR symptom score was performed. After successful modeling, mouse serum and nasal mucosa tissues were extracted for subsequent experiments. The expression levels of immunoglobulin E (IgE), Interleukin (IL)-4, IL-5, IL-13 and Tumor Necrosis Factor-α (TNF-α) in serum were detected using Enzyme-linked immunosorbent assay (ELISA); Hematoxylin–eosin (H&E) staining methods were used to observe the pathological changes of the nasal mucosal tissue; Utilizing immunohistochemistry (IHC) staining, the expression levels of TLR4, MyD88 and Nuclear factor kappa B (NF-κB) p65 in mouse nasal mucosa were quantified; The mRNA and protein expression levels of TLR4, MyD88 and NF-κB p65 in nasal mucosa of sensitized mice were detected with Quantitative reverse transcription PCR (qRT-PCR) and Western Blot. Finally, the in vitro culture of Human nasal mucosal epithelial cells (HNEpC) cells was conducted, and cells were treated with 200 µg/ml Artemisia annua pollen extract and OVA for 24 h. Western Blot assay was used to detect the expression level of TLR4, MyD88 and NF-κB p65 proteins before and after HNEpC cells were treated with MyD88 inhibitor ST-2825.
Result
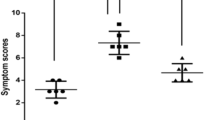
On the second day after AR stimulation, the mice showed obvious AR symptoms. H&E results showed that compared to the control group, the nasal mucosal tissue in the sensitized group was significantly more inflamed. Furthermore, ELISA assay showed increased expression levels of IgE, IL-4, IL-5, IL-13 and TNF-α in serum of mice induced by OVA and Artemisia annua pollen, Artemisia argyi pollen and Artemisia Sieversiana pollen than those of the control group. However, the expression level of IL-2 was lower than that of the control group (P < 0.05). Using Immunohistochemistry staining visually observed the expression levels of TLR4, MyD88 and NF-κB p65 in mouse nasal mucosa tissues and quantitatively analyzed. The expression levels of TLR4, MyD88 and NF-κB p65 in the sensitized group were higher than those in the control group, and the differences were statistically significant (P < 0.05). The results from qRT-PCR and Western Blot showed that the mRNA and protein expression levels of TLR4, MyD88 and NF-κB p65 in nasal mucosa of the sensitized group were significantly higher than those in the control group (P < 0.05). Finally, HNEpC cells were cultured in vitro and analyzed using Western Blot. The expression levels of TLR4, MyD88 and NF-κB p65 in OVA and An groups were significantly increased (P < 0.05). After ST-2825 treatment, TLR4 protein expression was significantly increased (P < 0.05) and MyD88 and NF-κB p65 protein expression were significantly decreased (P < 0.05).
Conclusion
To sum up, the occurrence and development of AR induced by OVA and pollen of Artemisia annua, Artemisia argyi and Artemisia Sieversiana were related to TLR4/MyD88 signal pathway.








Similar content being viewed by others
Data availability
The data of the present study are available from the corresponding author on reasonable request.
References:
Bousquet J et al (2020) Allergic rhinitis. Nat Rev Dis Prim 6(1):95
Xu X et al (2021) Comparative analysis of chronic rhinitis patient profiles during autumn pollen season between grassland and non-grassland cities in North China. Allergy Asthma Clin Immunol 17(1):106
Kim S et al (2023) Clinical characteristics of local allergic rhinitis sensitized to house dust mites in Asia. Eur Archiv Oto-Rhino-Laryngol. https://doi.org/10.1007/s00405-023-08394-y
Jaruvongvanich V et al (2016) Extranasal symptoms of allergic rhinitis are difficult to treat and affect quality of life. Allergol Int 65(2):199–203
Nakagome K, Nagata M (2021) Allergen immunotherapy in asthma. Pathogens 10(11):1406
Hughes KM, Price D, Suphioglu C (2022) Importance of allergen–environment interactions in epidemic thunderstorm asthma. Ther Adv Respir Dis 16:175346662210997
Amato DG et al (2007) Allergenic pollen and pollen allergy in Europe. Allergy 62(9):976–990
Roxbury CR et al (2018) Association between allergic rhinitis and poor sleep parameters in U.S. adults. Int Forum Allerg Rhinol 8(10):1098–1106
Sakashita M et al (2010) Prevalence of allergic rhinitis and sensitization to common aeroallergens in a Japanese population. Int Arch Allergy Immunol 151(3):255–261
Oberhuber C et al (2008) Prevalence of IgE-binding to Art v 1, Art v 4 and Amb a 1 in mugwort-allergic patients. Int Arch Allergy Immunol 145(2):94–101
Gao H et al (2022) Analysis of prevalence and risk factors of adult self-reported allergic rhinitis and asthma in plain lands and hilly areas of Shenmu city. China Front Public Health 9:749388
Tang R et al (2015) Artemisia allergy research in China. Biomed Res Int 2015:1–9
Soon L et al (2019) Therapeutic potential of Artemisia vulgaris: an insight into underlying immunological mechanisms. J Environ Pathol Toxicol Oncol 38(3):205–216
Wang Y et al (2022) Nonylphenol exacerbates ovalbumin-induced allergic rhinitis via the TSLP-TSLPR/IL-7R pathway and JAK1/2-STAT3 signaling in a mouse model. Ecotoxicol Environ Saf 243:114005
Adhikary PP et al (2021) TSLP as druggable target—a silver-lining for atopic diseases? Pharmacol Ther 217:107648
Nguyen TV et al (2022) Artemisia gmelinii extract alleviates allergic airway inflammation via balancing TH1/TH2 homeostasis and inhibiting mast cell degranulation. Int J Mol Sci 23(23):15377
Kang JH et al (2021) Lipopolysaccharide regulates thymic stromal lymphopoietin expression via TLR4/MAPK/Akt/NF-κB-signaling pathways in nasal fibroblasts: differential inhibitory effects of macrolide and corticosteroid. Int Forum Allerg Rhinol 11(2):144–152
Campion NJ et al (2023) Nasal IL-13 production identifies patients with late-phase allergic responses. J Allerg Clin Immunol. https://doi.org/10.1016/j.jaci.2023.06.026
Lee FFY, Alper S (2022) Alternative pre-mRNA splicing as a mechanism for terminating toll-like receptor signaling. Front Immunol 13:1023567
Zhang E, Ma Z, Lu M (2022) Contribution of T- and B-cell intrinsic toll-like receptors to the adaptive immune response in viral infectious diseases. Cell Mol Life Sci 79(11):547
Dong J et al (2021) Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol 43(3):319–327
Wei X et al (2023) TLR4-MyD88 signaling is involved in the spinal neurons during the full length of recovery from transection of the motor branch of the femoral nerve in mice. NeuroReport 34(13):655–663
Liu J et al (2020) MicroRNA-345-5p acts as an anti-inflammatory regulator in experimental allergic rhinitis via the TLR4/NF-κB pathway. Int Immunopharmacol 86:106522
Loiarro M et al (2007) Pivotal advance: inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J Leukoc Biol 82(4):801–810
Babayeva M et al (2022) A recombinant Artemisia vulgaris pollen adjuvanted Art v 1 protein-based vaccine treats allergic rhinitis and bronchial asthma using pre- and co-seasonal ultrashort immunotherapy regimens in sensitized mice. Front Immunol 13:983621
Brindisi G et al (2022) Allergic rhinitis, microbiota and passive smoke in children: a pilot study. Pediatr Allerg Immunol 33(S27):22–26
Pavón-Romero GF et al (2022) Allergen immunotherapy: current and future trends. Cells 11(2):212
Mai CW et al (2016) Mechanisms underlying the anti-inflammatory effects of clinacanthus nutans lindau extracts: inhibition of cytokine production and toll-like receptor-4 activation. Front Pharmacol 7:7
Zhou L et al (2017) Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep 7(1):44822
Su Q et al (2018) Effects of the TLR4/Myd88/NF-κB Signaling Pathway on NLRP3 Inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem 47(4):1497–1508
Ciprandi G et al (2015) From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol 11(12):1321–1333
Zhu Y et al (2023) Experimental observation of the effect of immunotherapy on CD4+ T cells and Th1/Th2 cytokines in mice with allergic rhinitis. Sci Rep 13(1):5273
Su B et al (2023) S14G-humanin ameliorates ovalbumin-induced airway inflammation in asthma mediated by inhibition of toll-like receptor 4 (TLR4) expression and the nuclear factor κ-B (NF-κB)/early growth response protein-1 (Egr-1) pathway. Aging 15(14):6822–6833
Tian X et al (2023) Probiotics alleviate food protein allergy in mice by activating TLR4 signaling pathway. Mol Nutr Food Res 67(12):e2200579
Zhao L et al (2020) Variation in IgE binding potencies of seven Artemisia species depending on content of major allergens. Clin Transl Allerg 10(1):50
Acknowledgements
The authors thank 51runse (www.51runse.com) for the English editing during the preparation of this manuscript.
Funding
This work was supported by the Research Project Supported by Shanxi Scholarship Council of China (2022–194);The First Hospital of Shanxi Medical University special fund research project, 136 special projects(Y2021136031);The First Hospital of Shanxi Medical University special fund research project, 136 special projects(Y2022136020).
Author information
Authors and Affiliations
Contributions
ZJ: designed the research and wrote the draft; GL: analyzed the experiment data; YDD and SYL: recorded animal behavior and provided data; FY: provided resources and supervised the entire work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethical Review Committee of experimental animal welfare in the first hospital of Shanxi Medical University (No:DWLL-2024–001). All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not application.
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Gao, L., Yu, D. et al. Three Artemisia pollens trigger the onset of allergic rhinitis via TLR4/MyD88 signaling pathway. Mol Biol Rep 51, 319 (2024). https://doi.org/10.1007/s11033-024-09350-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09350-7