Abstract
Background
Endothelial cells are crucial in maintaining the homeostasis of the blood-brain barrier. Girders of actin filament (Girdin) and phosphor (p)-Girdin are essential for the engulfment of human brain microvascular endothelial cells (HBMECs) into platelets (PLTs), but the potential mechanism remains unclear and requires further study.
Methods
Following PLT and cytochalasin D treatment, Hoechst 33,342 detected apoptosis. The transfection efficiency of the short hairpin RNA targeting Girdin (sh-Girdin) or overexpressing Girdin (OE-Girdin) was determined using western blotting. Sh-Girdin, OE-Girdin, mutated Girdin (m-Girdin), and microfilament binding region deleted Girdin (Del-Girdin) were transfected into HBMECs under PLT conditions. Subsequently, the engulfment of HBMECs by PLTs was detected by flow cytometry and transmission electron microscopy. Girdin and phosphorylated (p)-Girdin levels were quantified by western blot. The positive expression of Girdin was measured by immunohistochemistry (IHC). The localization of PLT, Girdin, and p-Girdin and the engulfment of HBMECs in PLTs were analyzed by confocal microscopy.
Result
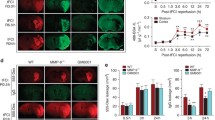
Cytochalasin D overturned the inhibitory effect of PLT on cell apoptosis. OE-Girdin enhanced the fluorescent intensity of PLT-labelling and the engulfment of HBMECs by PLTs, while sh-Girdin, m-Girdin, and Del-Girdin ran reversely. OE-Girdin elevated the Girdin and p-Girdin levels, while sh-Girdin and Del-Girdin were the opposite, but m-Girdin did not affect the p-Girdin and Girdin levels.
Conclusion
Girdin and p-Girdin were co-located with PLTs in HBMECs. The over-expression of Girdin was identified as being associated with the increasing engulfment of PTLs. Girdin may be an effective target to alleviate endothelial cell apoptosis.





Similar content being viewed by others
Data Availability
Data will be made available on reasonable request.
References
Knox EG, Aburto MR, Clarke G, Cryan JF, O’Driscoll CM (2022) The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry 27(6):2659–2673
Teleanu RI, Preda MD, Niculescu AG, Vladâcenco O, Radu CI, Grumezescu AM, Teleanu DM (2022) Current strategies to enhance delivery of drugs across the blood–brain barrier. Pharmaceutics 14(5):987
Trimm E, Red-Horse K (2023) Vascular endothelial cell development and diversity. Nat Reviews Cardiol 20(3):197–210
Sturtzel C (2017) Endothelial cells. Adv Exp Med Biol 1003:71–91
Jia G, Aroor AR, Jia C et al (2019) Endothelial cell senescence in aging-related vascular dysfunction. Biochim et Biophys acta Mol basis disease 1865(7):1802–1809
Sepúlveda C, Palomo I, Fuentes E (2017) Mechanisms of endothelial dysfunction during aging: predisposition to thrombosis. Mech Ageing Dev 164:91–99
Mussbacher M, Schossleitner K, Kral-Pointner JB, Salzmann M, Schrammel A, Schmid JA (2022) More than just a monolayer: the multifaceted role of endothelial cells in the pathophysiology of atherosclerosis. Curr Atheroscler Rep 24(6):483–492
Aron L, Zullo J, Yankner BA (2022) The adaptive aging brain. Curr Opin Neurobiol 72:91–100
Shevyrev D, Tereshchenko V, Berezina TN, Rybtsov S (2023) Hematopoietic stem cells and the Immune System in Development and Aging. Int J Mol Sci 24(6):5862
Blacher E, Tsai C, Litichevskiy L, Shipony Z, Iweka CA, Schneider KM, Chuluun B, Heller HC, Menon V, Thaiss CA, Andreasson KI (2022 Feb) Aging disrupts circadian gene regulation and function in macrophages. Nat Immunol 23(2):229–236
Peradinovic J, Mohovic N, Bulic K, Markovinovic A, Cimbro R, Munitic I (2023) Ageing-Induced decline in primary myeloid cell phagocytosis is unaffected by optineurin insufficiency. Biology 12(2):240
Duan W, Jin Y, Cui Y, Xi F, Liu X, Wo F, Wu J (2021) A co-delivery platform for synergistic promotion of angiogenesis based on biodegradable, therapeutic and self-reporting luminescent porous silicon microparticles. Biomaterials 272:120772
Catan A, Turpin C, Diotel N et al (2019) Aging and glycation promote erythrocyte phagocytosis by human endothelial cells: potential impact in atherothrombosis under diabetic conditions. Atherosclerosis 291:87–98
Kuckleburg CJ, McClenahan DJ, Czuprynski CJ (2008) Platelet activation by Histophilus somni and its lipooligosaccharide induces endothelial cell proinflammatory responses and platelet internalization. Shock (Augusta Ga) 29(2):189–196
Burlak C, Paris LL, Chihara RK et al (2010) The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation 17(5):350–361
Peng Q, Yeh H, Wei L et al (2012) Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS ONE 7(10):e47273
Seta Y, Kawakatsu K, Degawa S, Goto T, Nishikata T (2023) Morphological evidence for Novel Roles of Microtubules in Macrophage phagocytosis. Int J Mol Sci 24(2):1373
Paone S, Olivieri A (2022) Role of host small GTPases in Apicomplexan Parasite infection. Microorganisms 10(7):1370
Zhang Y, Liu X, Zhao J, Wang J, Song Q, Zhao C (2022) The phagocytic receptors of β-glucan. Int J Biol Macromol 205:430–441
Lalnunthangi A, Dakpa G, Tiwari S (2023) Multifunctional role of the ubiquitin proteasome pathway in phagocytosis. Prog Mol Biol Transl Sci 194:179–217
Cheng SY, Chen NF, Lin PY, Su JH, Chen BH, Kuo HM, Sung CS, Sung PJ, Wen ZH, Chen WF (2019) Anti-invasion and antiangiogenic effects of stellettin B through inhibition of the Akt/Girdin signaling pathway and VEGF in glioblastoma cells. Cancers 11(2):220
Pan S, Ren F, Li L, Liu D, Li Y, Wang A, Li W, Dong Y, Guo W (2020) MiR-328-3p inhibits cell proliferation and metastasis in colorectal cancer by targeting Girdin and inhibiting the PI3K/Akt signaling pathway. Exp Cell Res 390(1):111939
Wu L, Hua Y, Chang F, Kong D (2019) Girdin: an essential component of pre-replicative complex in human cells. bioRxiv. :634626
Lan Y, Li Y, Li D et al (2018) Engulfment of platelets delays endothelial cell aging via girdin and its phosphorylation. Int J Mol Med 42(2):988–997
Jiang P, Ren YL, Lan Y, Maywood et al (2015) NJ) 240(7):876–883
Wang W, Chen H, Gao W et al (2020) Girdin interaction with vimentin induces EMT and promotes the growth and metastasis of pancreatic ductal adenocarcinoma. Oncol Rep 44(2):637–649
Cortes-Canteli M, Iadecola C (2020) Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J Am Coll Cardiol 75(8):942–951
Li G, Yang T, Liu Y, Su H, Liu W, Fang D, Jin L, Jin F, Xu T, Duan C (2023) The proteins derived from platelet-rich plasma improve the endothelialization and vascularization of small diameter vascular grafts. Int J Biol Macromol 225:574–587
Fujita M, Horio T, Kishimoto S et al (2013) Effects of platelet-rich plasma-containing fragmin/protamine microparticles in enhancing endothelial and smooth muscle cell growth and inducing collateral vessels in a rabbit model of hindlimb ischemia. J biomedical Mater Res Part B Appl biomaterials 101(1):36–42
Ranzato E, Boccafoschi F, Mazzucco L et al (2010) Role of ERK1/2 in platelet lysate-driven endothelial cell repair. J Cell Biochem 110(3):783–793
Cho MS, Bottsford-Miller J, Vasquez HG et al (2012) Platelets increase the proliferation of ovarian cancer cells. Blood 120(24):4869–4872
Lang D, Dohle F, Terstesse M et al (1950) Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. Journal of immunology (Baltimore, Md: 2002,168(12):6152-8
Ji S, Dong W, Qi Y et al (2020) Phagocytosis by endothelial cells inhibits procoagulant activity of platelets of essential thrombocythemia in vitro. J Thromb haemostasis: JTH 18(1):222–233
Takahashi M, Asai N, Enomoto A (2015) Akt-girdin as oncotarget. Oncoscience 2(10):811–812
Miyake H, Maeda K, Asai N et al (2011) The actin-binding protein Girdin and its akt-mediated phosphorylation regulate neointima formation after vascular injury. Circul Res 108(10):1170–1179
Lansdown DA, Morrison C, Zaid MB et al (2019) Preoperative IDEAL (iterative decomposition of Echoes of asymmetrical length) magnetic resonance imaging rotator cuff muscle fat fractions are associated with rotator cuff repair outcomes. J Shoulder Elbow Surg 28(10):1936–1941
Funding
This work was supported by the National Natural Science Foundation of China no 31371160.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
The ethic committee of Beijing Hospital has reviewed and approved the conduction of our current study, with the written informed consent signed by each participant as well.
Consent to participate
Written informed consent was obtained from the participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, Y., Dong, M., Li, Y. et al. Upregulation of girdin delays endothelial cell apoptosis via promoting engulfment of platelets. Mol Biol Rep 50, 8111–8120 (2023). https://doi.org/10.1007/s11033-023-08625-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08625-9