Abstract
Background
Simvastatin can potentially mitigate acute inflammatory phase of myocardial ischemia-reperfusion injury. However, these effects negatively influenced by its poor bioavailability, low water solubility and high metabolism. Here, we investigated the effects of SIM-loaded nano-niosomes on a rat model of MI/R injury to find a drug delivery method to tackle the barriers.
Methods
Nano-niosomes’ characteristics were identified using dynamic light scattering and transmission electron microscopy. Fifty male Wistar rats were divided into five groups: Sham; MI/R; MI/R + nano-niosome; MI/R + SIM; MI/R + SIM-loaded nano-niosomes. Left anterior descending artery was ligated for 45 min, and 3 mg/kg SIM, nano-niosomes, or SIM-loaded nano-niosomes was intramyocardially injected ten min before the onset of reperfusion. ELISA assay was used to assess cardiac injury markers (cTnI, CK-MB) and inflammatory cytokines (TNF-α, IL-6, TGF-β, MPC-1). Expression level of MAPK-NF-κB and histopathological changes were evaluated by western blot and hematoxylin & eosin staining, respectively.
Results
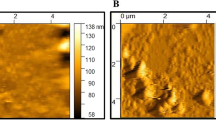
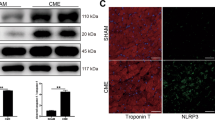
the size of nano-niosome was 137 nm, reached to 163 nm when simvastatin was loaded. To achieve optimized niosomes span 80, a drug/cholesterol ratio of 0.4 and seven min of sonication time was applied. Optimized entrapment efficiency of SIM-loaded nano-niosomes was 98.21%. Inflammatory cytokines and the expression level of MAPK and NF-κB were reduced in rats receiving SIM-loaded nano-niosomes compared to MI/R + SIM and MI/R + SIM-loaded nano-niosomes.
Conclusion
Our results showed that SIM-loaded nano-niosomes could act more efficiently than SIM in alleviating the acute inflammatory response of reperfusion injury via downregulating the activation of MAPK-NF-κB.




Similar content being viewed by others
Data Availability
All datasets generated are available on request.
Change history
26 April 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11033-023-08436-y
References
Safiri S et al (2022) Burden of ischemic heart disease and its attributable risk factors in 204 countries and territories, 1990–2019. Eur J Prev Cardiol 29(2):420–431
He J et al (2022) Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp Ther Med 23(6):430
Lodrini AM, Goumans MJ (2021) Cardiomyocytes Cellular Phenotypes After Myocardial Infarction. Front Cardiovasc Med 8:750510
Hausenloy DJ, Yellon DM (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Investig 123(1):92–100
Adameova A et al (2022) Interplay of Oxidative Stress and Necrosis-like Cell Death in Cardiac Ischemia/Reperfusion Injury: A Focus on Necroptosis.Biomedicines, 10(1)
Soares RO et al (2019) Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int J Mol Sci 20(20):5034
Duan J-S et al (2019) Urotensin-# receptor antagonist SB-706375 protected isolated rat heart from ischaemia–reperfusion injury by attenuating myocardial necrosis via RhoA/ROCK/RIP3 signalling pathway. Inflammopharmacology 27(6):1309–1318
Su X et al (2022) Mitochondrial Damage in Myocardial Ischemia/Reperfusion Injury and Application of Natural Plant Products. Oxid Med Cell Longev, 2022: p. 8726564
Mata A, Cadenas S (2021) The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia-Reperfusion Injury.Int J Mol Sci, 22(21)
Nazarinia D et al (2021) FoxO1 and Wnt/β-catenin signaling pathway: molecular targets of human amniotic mesenchymal stem cells-derived conditioned medium (hAMSC-CM) in protection against cerebral ischemia/reperfusion injury. J Chem Neuroanat 112:101918
Dong LY et al (2013) Cardioprotection of vitexin on myocardial ischemia/reperfusion injury in rat via regulating inflammatory cytokines and MAPK pathway. Am J Chin Med 41(6):1251–1266
Ma L et al (2014) Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-kappaB pathway: a mouse cardiomyocyte model. PLoS ONE 9(8):e103628
Ramli FF, Ali A, Ibrahim N (2022) Molecular-Signaling Pathways of Ginsenosides Rb in Myocardial Ischemia-Reperfusion Injury: A Mini Review. Int J Med Sci 19(1):65–73
Chen Z et al (2022) Inhibition of Myocardial Cell Apoptosis Is Important Mechanism for Ginsenoside in the Limitation of Myocardial Ischemia/Reperfusion Injury. Front Pharmacol 13:806216
Sadrkhanloo M et al (2022) Targeting Nrf2 in ischemia-reperfusion alleviation: From signaling networks to therapeutic targeting. Life Sci 300:120561
Khan SI et al (2017) Febuxostat Modulates MAPK/NF-kappaBp65/TNF-alpha Signaling in Cardiac Ischemia-Reperfusion Injury. Oxid Med Cell Longev 2017:8095825
Cai Z et al (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16(1):55–65
Byrne P et al (2019) Statins for the primary prevention of cardiovascular disease: an overview of systematic reviews. BMJ open 9(4):e023085
Lee MM et al (2019) Statins in the prevention and treatment of heart failure: a review of the evidence. Curr Atheroscler Rep 21(10):1–8
Korani S et al (2019) Parenteral systems for statin delivery: a review. Lipids Health Dis 18(1):1–9
De Angelis G (2004) The influence of statin characteristics on their safety and tolerability. Int J Clin Pract 58(10):945–955
Liu J, Shen Q, Wu Y (2008) Simvastatin prevents cardiac hypertrophy in vitro and in vivo via JAK/STAT pathway. Life Sci 82(19–20):991–996
Climent E, Benaiges D, Pedro-Botet J (2021) Hydrophilic or lipophilic statins? Front Cardiovasc Med 8:491
Wang X, Chen J, Huang X (2019) Rosuvastatin Attenuates Myocardial Ischemia-Reperfusion Injury via Upregulating miR-17-3p-Mediated Autophagy. Cell Reprogram 21(6):323–330
Wolfrum S et al (2004) Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase/Akt pathway. J Cardiovasc Pharmacol 44(3):348–355
Rizvi SZH et al (2019) Simvastatin-loaded solid lipid nanoparticles for enhanced anti-hyperlipidemic activity in hyperlipidemia animal model. Int J Pharm 560:136–143
Luo K, Long, Xu B-c (2015) Reduced apoptosis after acute myocardial infarction by simvastatin. Cell Biochem Biophys 71(2):735–740
Nagaoka K et al (2015) A new therapeutic modality for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin induces cardioprotection from ischemia-reperfusion injury via activation of PI3K/Akt pathway and anti-inflammation in a rat model. PLoS ONE 10(7):e0132451
Moghassemi S, Hadjizadeh A (2014) Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Controlled Release 185:22–36
Naderi R et al (2021) Preparation and evaluation of crocin loaded in nanoniosomes and their effects on ischemia–reperfusion injuries in rat kidney. Sci Rep 11(1):1–12
Sharma A et al (2019) Niosomes: a promising approach in drug delivery systems. J Drug Delivery Ther 9(4):635–642
Naseroleslami M et al (2022) Simvastatin-loaded nano-niosomes confer cardioprotection against myocardial ischemia/reperfusion injury. Drug Delivery and Translational Research 12(6):1423–1432
He C et al (2010) Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31(13):3657–3666
Sharifi M et al (2021) Necroptosis and RhoA/ROCK pathways: molecular targets of Nesfatin-1 in cardioprotection against myocardial ischemia/reperfusion injury in a rat model. Mol Biol Rep 48(3):2507–2518
Rakhshan K et al (2019) ELABELA (ELA) peptide exerts cardioprotection against myocardial infarction by targeting oxidative stress and the improvement of heart function. Int J Pept Res Ther 25(2):613–621
Naseroleslami M et al (2020) Nesfatin-1 attenuates injury in a rat model of myocardial infarction by targeting autophagy, inflammation, and apoptosis. Archives of Physiology and Biochemistry, pp 1–9
Kheila M et al (2021) Human mesenchymal stem cells derived from amniotic membrane attenuate isoproterenol (ISO)-induced myocardial injury by targeting apoptosis. Med J Islamic Repub Iran 35:82
Firozian F et al (2020) Improvement of therapeutic potential N-acetylcysteine in acetaminophen hepatotoxicity by encapsulation in PEGylated nano-niosomes. Life Sci 255:117832
Xu Y-Q et al (2016) Niosome encapsulation of curcumin: characterization and cytotoxic effect on ovarian cancer cells. Journal of Nanomaterials, 2016
Usman MRM, Ghuge PR, Jain BV (2017) Niosomes: a novel trend of drug delivery. Eur J Biomedical Pharm Sci 4(7):436–442
Verma A et al (2021) Emerging potential of niosomes in ocular delivery. Expert Opin Drug Deliv 18(1):55–71
Ge X et al (2019) Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 11(2):55
Tuuminen R et al (2016) Simvastatin pretreatment reduces caspase-9 and RIPK1 protein activity in rat cardiac allograft ischemia-reperfusion. Transpl Immunol 37:40–45
de Jesus Oliveira FM et al (2020) Simvastatin Posttreatment Controls Inflammation and Improves Bacterial Clearance in Experimental Sepsis. Mediators Inflamm, 2020: p. 1839762
Hadi NR et al (2013) Antiapoptotic effect of simvastatin ameliorates myocardial ischemia/reperfusion injury. International Scholarly Research Notices, 2013
Vilahur G et al (2009) Induction of RISK by HMG-CoA reductase inhibition affords cardioprotection after myocardial infarction. Atherosclerosis 206(1):95–101
Du Y et al (2018) Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome. Am J translational Res 10(12):4235
Qiao S et al (2021) Necrostatin-1 Analog DIMO Exerts Cardioprotective Effect against Ischemia Reperfusion Injury by Suppressing Necroptosis via Autophagic Pathway in Rats. Pharmacology 106(3–4):189–201
Razavi Tousi SMT et al (2022) Mesenchymal Stem Cells Derived from Human Amniotic Membrane Increase VEGF and Extenuate Fibrosis in Heart Failure Rats.Iranian Journal of Science and Technology, Transactions A: Science, : p.1–11
Bai R et al (2017) Corydalis hendersonii Hemsl. protects against myocardial injury by attenuating inflammation and fibrosis via NF-κB and JAK2-STAT3 signaling pathways. J Ethnopharmacol 207:174–183
Wang X et al (2022) Simvastatin Combined with Resistance Training Improves Outcomes in Patients with Chronic Heart Failure by Modulating Mitochondrial Membrane Potential and the Janus Kinase/Signal Transducer and Activator of Transcription 3 Signaling Pathways. Cardiovasc Ther, 2022: p. 8430733
Zhang J et al (2005) Simvastatin regulates myocardial cytokine expression and improves ventricular remodeling in rats after acute myocardial infarction. Cardiovasc Drugs Ther 19(1):13–21
Garg S, Bhattia J (2018) Fisetin, a PPAR gamma agonist improves myocardial injury in rats through Inhibition of MAPK Signalling Pathway mediated oxidative stress and inflammation in Experimental Model of Myocardial Ischemia Reperfusion Injury. in Proceedings for Annual Meeting of The Japanese Pharmacological Society WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology). Japanese Pharmacological Society
Zhou QL et al (2018) FPR1 gene silencing suppresses cardiomyocyte apoptosis and ventricular remodeling in rats with ischemia/reperfusion injury through the inhibition of MAPK signaling pathway. Exp Cell Res 370(2):506–518
Xu L et al (2022) Simvastatin inhibits the inflammation and oxidative stress of human neutrophils in sepsis via autophagy induction.Mol Med Rep, 25(1)
Hua Y et al (2018) Evaluation of effect of atorvastatin on left ventricular systolic function in rats with myocardial infarction via 2D-STI technique. Experimental and Therapeutic Medicine 15(5):4386–4394 .Statements & Declarations
Acknowledgements
This study was funded by a research grant from the Physiology Research Center, Iran University of Medical Science, Tehran, Iran.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed by [Nahid Aboutaleb]. [Masoomeh Sharifi] and [Maryam Naseroleslami] performed the animal model and experimental study. Data analyses and interpretation were accomplished by [Neda Mousavi Niri]. The first draft of the manuscript was written and proofread by [Nahid Aboutaleb] and [Masoomeh Sharifi]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Guidelines for using animals in this study were approved by the Committee of the Care and Use of Laboratory Animals at Iran University of Medical Sciences, ethical number: IR.IUMS.REC.1398.625. The Committee is credited by the Ministry of Health and Medical Education, which is initially works on the basis of The Declaration of Helsinki, the fifth revision.
Additional information
Maryam Naseroleslami and Masoomeh Sharifi authors have equally contributed to this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s11033-023-08436-y
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naseroleslami, M., Sharifi, M., Mousavi Niri, N. et al. RETRACTED ARTICLE: Simvastatin-loaded nano-niosomes efficiently downregulates the MAPK-NF-κB pathway during the acute phase of myocardial ischemia-reperfusion injury. Mol Biol Rep 49, 10377–10385 (2022). https://doi.org/10.1007/s11033-022-07891-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07891-3