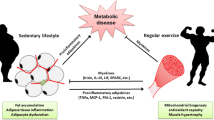
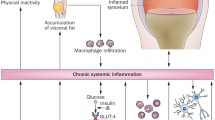
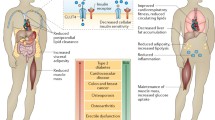
Abstract
Skeletal muscle has a robust endocrine function as a powerful organ and can secrete and release cytokines or polypeptides known as myokines. These myokines have significant regulatory effects on signal transduction in skeletal muscle and the metabolism of peripheral tissues and organs and exert biological effects via autocrine, paracrine, or endocrine forms. Obesity and aging cause myokine secretion dysregulation, and hastening sarcopenic obesity (SO) development. Exercise is currently an excellent intervention and prevention method for SO. Meanwhile, exercise impacts many organs and tissues. These organs and tissues will produce various myokines in response to movement and metabolism throughout the body to govern muscle differentiation, growth, and remodeling. According to accumulating data, exercise can increase the release of myokines from diverse tissues into the blood and postpone the SO onset and progression by influencing protein metabolism, inflammation, mitochondrial quality control, and other mechanisms.

Similar content being viewed by others
References
Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER 3, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS (2016) Effects of Testosterone Treatment in Older Men. N Engl J Med 374:611–624. https://doi.org/10.1056/NEJMoa1506119
Zilberrosenberg I (1989) Summary comments and methodological problems in determining nutritional status of older persons
Baumgartner RN (2000) Body composition in healthy aging. Ann N Y Acad Sci 904:437–448. https://doi.org/10.1111/j.1749-6632.2000.tb06498.x
Karstoft K, Pedersen BK (2016) Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care 19:270–275. https://doi.org/10.1097/mco.0000000000000283
Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M (2015) Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6:6670. https://doi.org/10.1038/ncomms7670
Roseno SL, Davis PR, Bollinger LM, Powell JJ, Witczak CA, Brault JJ (2015) Short-term, high-fat diet accelerates disuse atrophy and protein degradation in a muscle-specific manner in mice. Nutr Metab (Lond) 12:39. https://doi.org/10.1186/s12986-015-0037-y
Eckstein SS, Weigert C, Lehmann R (2017) Divergent roles of IRS (iInsulin receptor substrate) 1 and 2 in liver and skeletal muscle. Curr Med Chem 24:1827–1852. https://doi.org/10.2174/0929867324666170426142826
Boura-Halfon S, Zick Y (2009) Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab 296:E581–591. https://doi.org/10.1152/ajpendo.90437.2008
Mori RC, Poças da Silva T, Campello RS, Machado UF (2019) Carbenoxolone enhances peripheral insulin sensitivity and GLUT4 expression in skeletal muscle of obese rats: Potential participation of UBC9 protein. Life Sci 229:157–165. https://doi.org/10.1016/j.lfs.2019.05.017
Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C (2008) The developmental origins of sarcopenia. J Nutr Health Aging 12:427–432. https://doi.org/10.1007/bf02982703
Weinsier RL, Schutz Y, Bracco D (1992) Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am J Clin Nutr 55:790–794. https://doi.org/10.1093/ajcn/55.4.790
Sakuma K, Yamaguchi A (2013) Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol 2013:204164. https://doi.org/10.1155/2013/204164
Hood DA, Memme JM, Oliveira AN, Triolo M (2019) Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol 81:19–41. https://doi.org/10.1146/annurev-physiol-020518-114310
Aon MA, Bhatt N, Cortassa SC (2014) Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol 5:282. https://doi.org/10.3389/fphys.2014.00282
Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C (2013) Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 45:2288–2301. https://doi.org/10.1016/j.biocel.2013.06.024
Möhlig M, Isken F, Ristow M (2004) Impaired mitochondrial activity and insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:2419–2421 author reply 2419–2421
Hafizi Abu Bakar M, Kian Kai C, Wan Hassan WN, Sarmidi MR, Yaakob H, Zaman Huri H (2015) Mitochondrial dysfunction as a central event for mechanisms underlying insulin resistance: the roles of long chain fatty acids. Diabetes Metab Res Rev 31:453–475. https://doi.org/10.1002/dmrr.2601
Katzmarzyk PT, Janssen I, Ardern CI (2003) Physical inactivity, excess adiposity and premature mortality. Obes Rev 4:257–290. https://doi.org/10.1046/j.1467-789x.2003.00120.x
Kwon JH, Moon KM, Min KW (2020) Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthc (Basel) 8. https://doi.org/10.3390/healthcare8040378
Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A, Eckardt K (2014) The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun 450:1089–1094. https://doi.org/10.1016/j.bbrc.2014.06.123
Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M (2013) Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports 23:e150–161. https://doi.org/10.1111/j.1600-0838.2011.01414.x
Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ (2013) Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol 32:3–13. https://doi.org/10.1016/j.matbio.2012.11.005
El Shafey N, Guesnon M, Simon F, Deprez E, Cosette J, Stockholm D, Scherman D, Bigey P, Kichler A (2016) Inhibition of the myostatin/Smad signaling pathway by short decorin-derived peptides. Exp Cell Res 341:187–195. https://doi.org/10.1016/j.yexcr.2016.01.019
Magarò MS, Bertacchini J, Florio F, Zavatti M, Potì F, Cavani F, Amore E, De Santis I, Bevilacqua A, Reggiani Bonetti L, Torricelli P, Maurel DB, Biressi S, Palumbo C (2021) Identification of Sclerostin as a Putative New Myokine Involved in the Muscle-to-Bone Crosstalk. https://doi.org/10.3390/biomedicines9010071. Biomedicines 9
Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, Mohanty ST, Croucher P, Kramer I, Kneissel M, Rosen CJ, Reagan MR (2018) The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol 233:1156–1167. https://doi.org/10.1002/jcp.25976
Ukita M, Yamaguchi T, Ohata N, Tamura M (2016) Sclerostin Enhances Adipocyte Differentiation in 3T3-L1 Cells. J Cell Biochem 117:1419–1428. https://doi.org/10.1002/jcb.25432
Fulzele K, Lai F, Dedic C, Saini V, Uda Y, Shi C, Tuck P, Aronson JL, Liu X, Spatz JM, Wein MN, Divieti Pajevic P (2017) Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J Bone Miner Res 32:373–384. https://doi.org/10.1002/jbmr.3001
Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, Da H, Aja S, Noh HL, Kim JK, Hussain MA, Thorek DLJ, Wolfgang MJ, Riddle RC (2017) Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A 114:E11238–e11247. https://doi.org/10.1073/pnas.1707876115
Ardawi MS, Rouzi AA, Qari MH (2012) Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab 97:3691–3699. https://doi.org/10.1210/jc.2011-3361
Hinton PS, Nigh P, Thyfault J (2017) Serum sclerostin decreases following 12months of resistance- or jump-training in men with low bone mass. Bone 96:85–90. https://doi.org/10.1016/j.bone.2016.10.011
Pickering ME, Simon M, Sornay-Rendu E, Chikh K, Carlier MC, Raby AL, Szulc P, Confavreux CB (2017) Serum Sclerostin Increases After Acute Physical Activity. Calcif Tissue Int 101:170–173. https://doi.org/10.1007/s00223-017-0272-5
Falk B, Haddad F, Klentrou P, Ward W, Kish K, Mezil Y, Radom-Aizik S (2016) Differential sclerostin and parathyroid hormone response to exercise in boys and men. Osteoporos Int 27:1245–1249. https://doi.org/10.1007/s00198-015-3310-z
Sharma-Ghimire P, Chen Z, Sherk V, Bemben M, Bemben D (2019) Sclerostin and parathyroid hormone responses to acute whole-body vibration and resistance exercise in young women. J Bone Miner Metab 37:358–367. https://doi.org/10.1007/s00774-018-0933-0
Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, Qualls C, Villareal DT (2012) Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res 27:1215–1221. https://doi.org/10.1002/jbmr.1560
Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, Kim SJ, Choi JI, Oh Y, Kim JH, Suyeon H, Park SH, Kim HS (2015) Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol 29:873–881. https://doi.org/10.1210/me.2014-1353
Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, Sharma M, Kambadur R (2017) Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun 8:1104. https://doi.org/10.1038/s41467-017-01131-0
Liu J, Song N, Huang Y, Chen Y (2018) Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci Rep 8:15247. https://doi.org/10.1038/s41598-018-33229-w
Piccirillo R (2019) Exercise-induced myokines with therapeutic potential for muscle wasting. Front Physiol 10:287. https://doi.org/10.3389/fphys.2019.00287
Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisløff U, Tjønna AE, Raastad T, Hallén J, Norheim F, Drevon CA, Romacho T, Eckardt K, Eckel J (2013) Evidence against a beneficial effect of irisin in humans. PLoS ONE 8:e73680. https://doi.org/10.1371/journal.pone.0073680
McFarlane C, Hui GZ, Amanda WZ, Lau HY, Lokireddy S, Xiaojia G, Mouly V, Butler-Browne G, Gluckman PD, Sharma M, Kambadur R (2011) Human myostatin negatively regulates human myoblast growth and differentiation. Am J Physiol Cell Physiol 301:C195–203. https://doi.org/10.1152/ajpcell.00012.2011
Pistilli EE, Bogdanovich S, Goncalves MD, Ahima RS, Lachey J, Seehra J, Khurana T (2011) Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. Am J Pathol 178:1287–1297. https://doi.org/10.1016/j.ajpath.2010.11.071
Han HQ, Zhou X, Mitch WE, Goldberg AL (2013) Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol 45:2333–2347. https://doi.org/10.1016/j.biocel.2013.05.019
Lee JH, Jun HS (2019) Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front Physiol 10:42. https://doi.org/10.3389/fphys.2019.00042
Huang Z, Chen X, Chen D (2011) Myostatin: a novel insight into its role in metabolism, signal pathways, and expression regulation. Cell Signal 23:1441–1446. https://doi.org/10.1016/j.cellsig.2011.05.003
Walker KS, Kambadur R, Sharma M, Smith HK (2004) Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc 36:787–793. https://doi.org/10.1249/01.mss.0000126384.04778.29
Ko IG, Jeong JW, Kim YH, Jee YS, Kim SE, Kim SH, Jin JJ, Kim CJ, Chung KJ (2014) Aerobic exercise affects myostatin expression in aged rat skeletal muscles: a possibility of antiaging effects of aerobic exercise related with pelvic floor muscle and urethral rhabdosphincter. Int Neurourol J 18:77–85. https://doi.org/10.5213/inj.2014.18.2.77
Baati N, Feillet-Coudray C, Fouret G, Vernus B, Goustard B, Jollet M, Bertrand-Gaday C, Coudray C, Lecomte J, Bonnieu A, Koechlin-Ramonatxo C (2019) New evidence of exercise training benefits in myostatin-deficient mice: Effect on lipidomic abnormalities. Biochem Biophys Res Commun 516:89–95. https://doi.org/10.1016/j.bbrc.2019.06.014
Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, Daviaud D, Mir L, Marques MA, Thalamas C, Valet P, Langin D, Moro C, Viguerie N (2014) Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (Lond) 38:707–713. https://doi.org/10.1038/ijo.2013.158
Son JS, Kim HJ, Son Y, Lee H, Chae SA, Seong JK, Song W (2017) Effects of exercise-induced apelin levels on skeletal muscle and their capillarization in type 2 diabetic rats. Muscle Nerve 56:1155–1163. https://doi.org/10.1002/mus.25596
Castan-Laurell I, Dray C, Knauf C, Kunduzova O, Valet P (2012) Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol Metab 23:234–241. https://doi.org/10.1016/j.tem.2012.02.005
Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradère JP, Le Gonidec S, Dortignac A, Geoffre N, Pereira O, Karaz S, Lee U, Camus M, Chaoui K, Mouisel E, Bigot A, Mouly V, Vigneau M, Pagano AF, Chopard A, Pillard F, Guyonnet S, Cesari M, Burlet-Schiltz O, Pahor M, Feige JN, Vellas B, Valet P, Dray C (2018) The exerkine apelin reverses age-associated sarcopenia. Nat Med 24:1360–1371. https://doi.org/10.1038/s41591-018-0131-6
Kwak SE, Cho SC, Bae JH, Lee J, Shin HE, Di Zhang D, Lee YI, Song W (2019) Effects of exercise-induced apelin on muscle function and cognitive function in aged mice. Exp Gerontol 127:110710. https://doi.org/10.1016/j.exger.2019.110710
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE (2014) β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19:96–108. https://doi.org/10.1016/j.cmet.2013.12.003
Jung TW, Park HS, Choi GH, Kim D, Lee T (2018) β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J Biomed Sci 25:27. https://doi.org/10.1186/s12929-018-0431-7
Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM (2015) BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARδ-dependent pathway in mice. Diabetologia 58:2096–2105. https://doi.org/10.1007/s00125-015-3663-z
Stautemas J, Van Kuilenburg ABP, Stroomer L, Vaz F, Blancquaert L, Lefevere FBD, Everaert I, Derave W (2019) Acute aerobic exercise leads to increased plasma levels of R- and S-β-aminoisobutyric acid in humans. Front Physiol 10:1240. https://doi.org/10.3389/fphys.2019.01240
Short KR, Chadwick JQ, Teague AM, Tullier MA, Wolbert L, Coleman C, Copeland KC (2019) Effect of obesity and exercise training on plasma amino acids and amino metabolites in american indian adolescents. J Clin Endocrinol Metab 104:3249–3261. https://doi.org/10.1210/jc.2018-02698
Lewitt MS, Boyd GW (2019) The Role of Insulin-Like Growth Factors and Insulin-Like Growth Factor-Binding Proteins in the Nervous System. Biochem insights 12:1178626419842176. https://doi.org/10.1177/1178626419842176
Clemmons DR (2009) Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab 20:349–356. https://doi.org/10.1016/j.tem.2009.04.002
McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G (1999) Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516(Pt 2):583–592. https://doi.org/10.1111/j.1469-7793.1999.0583v.x
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013. https://doi.org/10.1038/ncb1101-1009
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984. https://doi.org/10.1016/j.biocel.2005.04.018
Urso ML, Fiatarone Singh MA, Ding W, Evans WJ, Cosmas AC, Manfredi TG (2005) Exercise training effects on skeletal muscle plasticity and IGF-1 receptors in frail elders. Age (Dordr) 27:117–125. https://doi.org/10.1007/s11357-005-1629-7
Apel PJ, Ma J, Callahan M, Northam CN, Alton TB, Sonntag WE, Li Z (2010) Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve 41:335–341. https://doi.org/10.1002/mus.21485
Chen H, Weber AJ (2004) Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Res 1011:99–106. https://doi.org/10.1016/j.brainres.2004.03.024
Yarrow JF, White LJ, McCoy SC, Borst SE (2010) Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett 479:161–165. https://doi.org/10.1016/j.neulet.2010.05.058
Delezie J, Weihrauch M, Maier G, Tejero R, Ham DJ, Gill JF, Karrer-Cardel B, Rüegg MA, Tabares L, Handschin C (2019) BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc Natl Acad Sci U S A 116:16111–16120. https://doi.org/10.1073/pnas.1900544116
Tsai CL, Pan CY, Chen FC, Wang CH, Chou FY (2016) Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Exp Physiol 101:836–850. https://doi.org/10.1113/ep085682
Saucedo Marquez CM, Vanaudenaerde B, Troosters T, Wenderoth N (2015) High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol (1985) 119:1363–1373. https://doi.org/10.1152/japplphysiol.00126.2015
Zhang ZY, Mai Y, Yang H, Dong PY, Zheng XL, Yang GS (2014) CTSB promotes porcine preadipocytes differentiation by degrading fibronectin and attenuating the Wnt/β-catenin signaling pathway. Mol Cell Biochem 395:53–64. https://doi.org/10.1007/s11010-014-2111-6
Mizunoe Y, Sudo Y, Okita N, Hiraoka H, Mikami K, Narahara T, Negishi A, Yoshida M, Higashibata R, Watanabe S, Kaneko H, Natori D, Furuichi T, Yasukawa H, Kobayashi M, Higami Y (2017) Involvement of lysosomal dysfunction in autophagosome accumulation and early pathologies in adipose tissue of obese mice. Autophagy 13:642–653. https://doi.org/10.1080/15548627.2016.1274850
Gornicka A, Fettig J, Eguchi A, Berk MP, Thapaliya S, Dixon LJ, Feldstein AE (2012) Adipocyte hypertrophy is associated with lysosomal permeability both in vivo and in vitro: role in adipose tissue inflammation. Am J Physiol Endocrinol Metab 303:E597–606. https://doi.org/10.1152/ajpendo.00022.2012
Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H (2016) Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab 24:332–340. https://doi.org/10.1016/j.cmet.2016.05.025
Yang YR, Kwon KS (2020) Potential Roles of Exercise-Induced Plasma Metabolites Linking Exercise to Health Benefits. Front Physiol 11:602748. https://doi.org/10.3389/fphys.2020.602748
Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F, Casteilla L (2014) Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63:3253–3265. https://doi.org/10.2337/db13-1885
Hoshino D, Yoshida Y, Kitaoka Y, Hatta H, Bonen A (2013) High-intensity interval training increases intrinsic rates of mitochondrial fatty acid oxidation in rat red and white skeletal muscle. Appl Physiol Nutr Metab 38:326–333. https://doi.org/10.1139/apnm-2012-0257
Zwaag J, Ter Horst R, Blaženović I, Stoessel D, Ratter J, Worseck JM, Schauer N, Stienstra R, Netea MG, Jahn D, Pickkers P, Kox M (2020) Involvement of Lactate and Pyruvate in the Anti-Inflammatory Effects Exerted by Voluntary Activation of the Sympathetic Nervous System. https://doi.org/10.3390/metabo10040148. Metabolites 10
Steinman MQ, Gao V, Alberini CM (2016) The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Front Integr Neurosci 10:10. https://doi.org/10.3389/fnint.2016.00010
Nikooie R, Samaneh S (2016) Exercise-induced lactate accumulation regulates intramuscular triglyceride metabolism via transforming growth factor-β1 mediated pathways. Mol Cell Endocrinol 419:244–251. https://doi.org/10.1016/j.mce.2015.10.024
Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Nigro P, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee MY, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P, Kalliokoski KK, Nielsen S, Pedersen BK, Kahn CR, Tseng YH, Goodyear LJ (2019) TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 1:291–303. https://doi.org/10.1038/s42255-018-0030-7
Keipert S, Ost M, Johann K, Imber F, Jastroch M, van Schothorst EM, Keijer J, Klaus S (2014) Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am J Physiol Endocrinol Metab 306:E469–482. https://doi.org/10.1152/ajpendo.00330.2013
Kim CS, Joe Y, Choi HS, Back SH, Park JW, Chung HT, Roh E, Kim MS, Ha TY, Yu R (2019) Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle. J Inflamm (Lond) 16:17. https://doi.org/10.1186/s12950-019-0221-3
Taniguchi H, Tanisawa K, Sun X, Kubo T, Higuchi M (2016) Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J Clin Endocrinol Metab 101:191–198. https://doi.org/10.1210/jc.2015-3308
Khalafi M, Alamdari KA, Symonds ME, Nobari H, Carlos-Vivas J (2021) Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: systematic review and meta-analysis. Horm (Athens) 20:23–33. https://doi.org/10.1007/s42000-020-00245-3
Brandt C, Pedersen BK (2010) The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol 2010:520258. https://doi.org/10.1155/2010/520258
Quinn LS, Strait-Bodey L, Anderson BG, Argilés JM, Havel PJ (2005) Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int 29:449–457. https://doi.org/10.1016/j.cellbi.2005.02.005
Krolopp JE, Thornton SM, Abbott MJ (2016) IL-15 activates the Jak3/STAT3 signaling pathway to mediate glucose uptake in skeletal muscle cells. Front Physiol 7:626. https://doi.org/10.3389/fphys.2016.00626
Yalcin A, Silay K, Balik AR, Avcioğlu G, Aydin AS (2018) The relationship between plasma interleukin-15 levels and sarcopenia in outpatient older people. Aging Clin Exp Res 30:783–790. https://doi.org/10.1007/s40520-017-0848-y
Pistilli EE, Siu PM, Alway SE (2007) Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol 292:C1298–1304. https://doi.org/10.1152/ajpcell.00496.2006
Fujimoto T, Sugimoto K, Takahashi T, Yasunobe Y, Xie K, Tanaka M, Ohnishi Y, Yoshida S, Kurinami H, Akasaka H, Takami Y, Takeya Y, Yamamoto K, Rakugi H (2019) Overexpression of Interleukin-15 exhibits improved glucose tolerance and promotes GLUT4 translocation via AMP-Activated protein kinase pathway in skeletal muscle. Biochem Biophys Res Commun 509:994–1000. https://doi.org/10.1016/j.bbrc.2019.01.024
Pérez-López A, McKendry J, Martin-Rincon M, Morales-Alamo D, Pérez-Köhler B, Valadés D, Buján J, Calbet JAL, Breen L (2018) Skeletal muscle IL-15/IL-15Rα and myofibrillar protein synthesis after resistance exercise. Scand J Med Sci Sports 28:116–125. https://doi.org/10.1111/sms.12901
Rinnov A, Yfanti C, Nielsen S, Akerström TC, Peijs L, Zankari A, Fischer CP, Pedersen BK (2014) Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine 45:271–278. https://doi.org/10.1007/s12020-013-9969-z
Crane JD, MacNeil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, Kemp BE, Raha S, Steinberg GR, Tarnopolsky MA (2015) Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 14:625–634. https://doi.org/10.1111/acel.12341
Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, Kitamura T, Shimomura I (2004) Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem 279:19391–19395. https://doi.org/10.1074/jbc.C400066200
Toloza FJK, Mantilla-Rivas JO, Pérez-Matos MC, Ricardo-Silgado ML, Morales-Alvarez MC, Pinzón-Cortés JA, Pérez-Mayorga M, Arévalo-Garcia ML, Tolosa-González G, Mendivil CO (2018) Plasma levels of myonectin but not myostatin or fibroblast-ferived growth factor 21 are associated with insulin resistance in adult humans without diabetes mellitus. Front Endocrinol (Lausanne) 9:5. https://doi.org/10.3389/fendo.2018.00005
Seldin MM, Wong GW (2012) Regulation of tissue crosstalk by skeletal muscle-derived myonectin and other myokines. Adipocyte 1:200–202. https://doi.org/10.4161/adip.20877
Thyfault JP, Du M, Kraus WE, Levine JA, Booth FW (2015) Physiology of sedentary behavior and its relationship to health outcomes. Med Sci Sports Exerc 47:1301–1305. https://doi.org/10.1249/mss.0000000000000518
Eaton M, Granata C, Barry J, Safdar A, Bishop D, Little JP (2018) Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J Sport Health Sci 7:191–196. https://doi.org/10.1016/j.jshs.2017.01.003
Granata C, Jamnick NA, Bishop DJ (2018) Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med 48:1809–1828. https://doi.org/10.1007/s40279-018-0936-y
Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJ, Forouzanfar MH (2016) Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 354:i3857. https://doi.org/10.1136/bmj.i3857
Funding
This work was supported by the Graduate Research and Innovation Projects of Jiangsu Province of China (No. KYCX21_2909) and the Key Projects of Social Science Foundation of Jiangsu Province (No. 22TYA002).
Author information
Authors and Affiliations
Contributions
Zhang and Lv wrote and revised the paper and figure. Wang conducted the literature search. Ren and Yong guided the writing process and reviewed the manuscript. All authors critically reviewed progressive drafts of the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The author(s) declares no potential conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Zhang and Junjie Lv authors conducted the equal contribution to this project.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Lv, J., Wang, C. et al. Myokine, a key cytokine for physical exercise to alleviate sarcopenic obesity. Mol Biol Rep 50, 2723–2734 (2023). https://doi.org/10.1007/s11033-022-07821-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07821-3