Abstract
Background
Important risk factors for the most common sexually transmitted infection (STI) in the world, human papillomavirus (HPV), include early sexual activity, use of contraceptives, tobacco smoking, and immunological and genetic factors. This study aimed to investigate the relationship between GSTM1 and GSTT1 polymorphisms and HPV infection and associated risk factors in a group of women assisted in the public health system of southwestern Paraná, Brazil.
Methods and results
A case–control study was designed with 21 women with HPV matched by age in the case group and 84 women without the virus in the control group. Viral detection was conducted via polymerase chain reaction (PCR) and GSTM1 and GSTT1 genotyping by Multiplex PCR. The results showed that the GSTT1 null allele was a protective factor against infection (ORadj 0.219; 95% CI 0.078–0.618; p = 0.004). No relationship was observed for the GSTM1 gene. Smoking was defined as a risk factor (ORadj 3.678; 95% CI 1.111–12.171; p = 0.033), increasing the chances of HPV by up to 3.6 times.
Conclusion
This study showed, for the first time, the relationship between GSTM1 and GSTT1 genetic polymorphisms and HPV. We found that this relationship protected women from southern Brazil from viral infection, but not from susceptibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human papillomavirus (HPV) is a virus that can infect the skin and mucous membranes. It comprises about 220 distinct subtypes [1], out of which 40 can infect the anogenital tract, characterizing HPV as the most common sexually transmitted infection (STI) in the world [2]. Among the subtypes, 12 are defined as high risk for the development of cancer, including cervical cancer (CC), especially HPV-16 and HPV-18 [3]. In women, this type of cancer is the fourth most frequently diagnosed and the fourth leading cause of cancer death, with a global estimate of 604,000 new cases and 342,000 deaths in 2020 [4]. Although HPV infection is the main risk factor for CC, it is mostly an insufficient event if occurred alone [5].
Several factors significant in viral biology and disease progression are well defined in the literature, including ethnicity [6], early onset of sexual activity [7], use of contraceptives [8], exposure to tobacco [6, 9], co-infection with more than one viral subtype [10], oxidative stress [11], and immunological and genetic factors [12].
The cytosolic glutathione S-transferases (GSTs) belong to a family of enzymes involved in phase II of xenobiotic metabolism, being divided into eight gene classes: alfa (α), mu (μ), pi (π), theta (θ), sigma (σ), kappa (κ), omega (ω), and zeta (ζ) [13]. The GSTM1 and GSTT1 genes located on chromosome 1p13.3 and 22q11.23 exhibit insertion and deletion polymorphism, respectively, whereas the null allele characterizes the absence of enzymatic function [14]. This condition has been related to oxidative stress, which could be associated with pathologies including diabetes mellitus, prostate cancer [15], breast cancer [16], and CC [17]. It also favors the replication of pathogens, such as the human immunodeficiency virus (HIV), in different ethnic groups [18].
Despite gaps in the literature, evidence shows that genes involved in xenobiotic metabolism can affect the natural history of HPV subtypes, especially of those with high oncogenic risk [17]. Several studies indicate a relationship between GSTM1 and GSTT1 null genotypes and CC [19], but such relationship differs among populations and cannot be generalized [20]. An Indian study found a higher frequency of GSTM1 and GSTT1 null alleles in women with CC infected with the virus [21]. However, most studies prioritize the relationship between deleted alleles and viral infection events, not susceptibility [17, 22]. Sudenga et al. [17] found that HPV-16 infection was associated with null GSTM1 and GSTT1 genotypes, whereas deleted GSTM1 was associated with a lower persistence (or higher clearance) of the high-risk subtype.
A recent systematic review conducted by the authors of this research gathered studies that indirectly addressed the relationship between SNPs (single nucleotide polymorphisms), GSTM1 and GSTT1, and high-risk HPV infection in women with or without cervical alterations [23]. The study was inconclusive, but indicated a higher frequency of the GSTT1 null allele than of the deleted GSTM1 in women infected with high-risk HPV subtypes. Considering that no studies have been conducted on the direct relationship between SNPs and viral infection, an inconsistent subject, this study aimed to investigate the relationship between GSTM1 and GSTT1 polymorphisms and HPV infection and associated risk factors in a group of women assisted by the public health system in southwestern Paraná, Brazil.
Materials and methods
Study population and sampling
In 2017, 31,435 women aged 14–79 years were living in Francisco Beltrão. This number was used to estimate the study sample and determine the prevalence of cervical changes (Treco et al., 2021), exploring the prevalence of HPV infection in women under gynecological treatment in primary care units (UBS). The UBS included in the study were the family health strategy (FHS) units Antônio de Paiva Cantelmo, Cristo Rei, Industrial, and Pinheirinho. The Women’s Institute (Instituto da Mulher—IM), a reference center in outpatient gynecology and obstetrics care, was also included. Sample size was estimated with Epi Info version 7.2.2.6 (https://www.cdc.gov/epiinfo/support/por/pt_downloads.html), assuming a 25–30% prevalence of HPV infection. The power was set at 95%. The minimum sample size was 320 women and 10% more participants were added to avoid complications from losses and refusals, totaling 350 participants. All participants met the inclusion criteria of having had their first sexual intercourse at the time of the research, and pregnant women were excluded. Out of the 350 participants, 22 were diagnosed with HPV. Then, a case–control study was conducted with 105 women. Cases included women with HPV infection (n = 21; one participant was excluded since her genetic material did not allow characterizing genetic polymorphisms) whereas controls were formed by women without viral infection (n = 84). Cases and controls were matched by age (± 2 years), with four controls for each case.
Besides GSTM1 and GSTT1 polymorphism, other potential factors associated with HPV infection were assessed, including tobacco smoking, alcohol consumption, use of contraceptives, and vaginal infection, all with potential xenobiotic characteristics. Race was considered as a relevant variable for the investigated polymorphisms. All information on these variables were obtained by interviews conducted individually with a validated questionnaire [24].
During the gynecological consultation, women underwent material collection for the Pap smear. The endocervical brush used in the collection was placed in a microtube with 2 ml of saline solution kept at − 20 °C until virus detection [24]. The storage of biological material and extraction of genetic material, viral detection, and GST polymorphisms were conducted at the Molecular Biology Laboratory of the Universidade Estadual do Oeste do Paraná, in Francisco Beltrão.
The project was approved by the Research Ethics Committee involving Human Beings (REC) and the National Research Ethics Committee (CONEP), Opinion No. 2.254.450 and CAAE: 72983817.5.0000.0107. After receiving information about the research, the women who agreed to participate signed the Informed Consent Form (ICF) (Appendix I) to be included in the study.
Total DNA extraction and HPV detection
A 200 µl aliquot of the original sample was used to isolate the total genetic material following the extraction and purification protocol “Biological Fluid/Blood Genomic DNA extraction kit”—“Purelink® Genomic DNA Mini Kit” (Invitrogen by Thermo Fisher Scientific, Carlsbad, California) according to the manufacturer’s instructions and stored in a freezer at − 20 °C. For the molecular detection of HPV, specific primers were used for in vitro synthesis of the coding region of the virus’ L1 gene, MY09 (5′-CGTCCMAARGGAWACTGATC-3′) and MY11 (5′-GCMCAGGGWCATAAYAATGG-3′), producing a fragment of 450 bp. The final volume of each PCR reaction was 25 µl, with 10 mM Tris–HCl, 2 mM MgCl2, 0.1 mM dNTPs, 0.5 μM of each primer, 1.25 U of Taq DNA Polymerase (Ludwig Biotecnologia Taq DNA Polimerase, Brazil), and 3.5 µl of total DNA added at the end (50 ng/µl). To control the extraction and synthesis process, PCR of a 268 bp segment of the human β-globin gene was performed in all samples, using primers GH20 (5′-GAAGAGCCAAGGACAGGTAC-3′) and PCO4 (5′-CAACTTCATCCACGTTCACC-3′) [25]. The amplifications of both genomes were processed in the thermocycler Applied Biosystems Veriti Thermal Cycler (Thermo Fisher Scientific, Germany) in this sequence: 10 min at 94 °C, followed by 37 cycles of 1 min at 94 °C; 1 min at 55 °C and 1 min at 72 °C; finally, extension for 10 min at 72 °C [25]. As a positive control for viral detection, a DNA sample from HeLa cells (HPV-16) was included. All amplicons were fractionated via 2% agarose gel electrophoresis, stained with ethidium bromide, visualized under ultraviolet light (UV), and photodocumented.
GSTM1 and GSTT1 genotyping
Genotyping was performed by Multiplex PCR. The primer pairs used were 5′-GAACTCCCTGAAAAGCTAAAGC-3′ (forward) and 5′-GTTGGGCTCAAATA TACGGTGG-3′ (reverse) for GSTM1 and 5′-TTCCTTACTGGTCCTCACATCTC-3′ (forward) and 5′-TCACCGGATCATGGCCAGCA-3′ (reverse) for GSTT1. The first pair produces an amplicon of 219 bp and the second of 459 bp [26]. PCR conditions included initial denaturation at 94 °C for 5 min, followed by 35 cycles at 94 °C for 1 min, 58 °C for 1 min, 72 °C for 1 min, and 72 °C for 10 min. The genotypes were fractionated via 2% agarose gel electrophoresis, stained with ethidium bromide, visualized, and photodocumented. The presence of amplicons characterizes the functional alleles and the absence of null alleles.
Statistical analysis
Genotypic frequencies, GSTM1 and GSTT1, and the variables tobacco smoking, alcohol consumption, use of contraceptives, vaginal infection, and race were determined and compared between cases and controls using the chi-squared test (χ2), with Yates’ and Fisher’s correction for continuity. Variables with a significance value smaller than 0.20 were used for multivariate logistic regression, with a 95% confidence interval and p < 0.05, to determine the risk factors for STIs. All statistical analyzes were performed using the Statistical Package for the Social Sciences (SPSS) software version 24.0.
Results
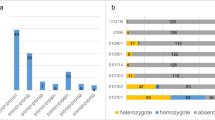
Figure 1 shows the genotypic pattern of GSTM1 and GSTT1 genes and Table 1 shows the allele frequency spectrum. The GSTM1 null allele (GSTM1−) was more frequent in both cases (57.1%) and controls (75.0%). On the other hand, the GSTT1 null allele (GSTT1−) was less frequent (38.1%) in the case group than the GSTT1+ allele (61.9%) and more frequent in the control group (73.8%). Furthermore, the most common allelic combination was GSTM1−/GSTT1+ in the case group and double deletion in the control group.
Electrophoresis of PCR products from the co-amplification of GSTM1 and GSTT1. 2% agarose gel electrophoresis stained with ethidium bromide. Lanes (L): L1–5, L7, L9 (A) were positive for GSTM1; L6, L8, L13, L14 (A), and L18, L19, and L22 (B) were GSTM1 and GSTT1 deletion genotypes. Lanes L11, L12 (A), and L21 and L23 (B) were positive for GSTM1 and GSTT1; L20 were positive for GSTM1 genotype (B). Lanes L15 and L17 were negative control (A and B) and L10 and L16 were 100 bp molecular weight markers (A and B)
Table 2 shows the results of the bivariate analysis (chi-squared test, χ2), suggesting associations between GST polymorphisms, life habits, vaginal infection, and race among groups. The choice of variables was based on the possible relationship with xenobiotic metabolism, which is directly associated with GST genes. All categories established for GST polymorphism (GSTM1, p = 0.177; GSTT1, p = 0.004; Combinations, p = 0.002) and smoking (p = 0.040), recent vaginal infection (p = 0.132), and race (p = 0.043) categories suggested associations with HPV.
Although the use of contraceptives and the consumption of alcohol showed no significant association with the virus, 81% of women with HPV use or have used the medication and 61.9% consume some type of alcoholic beverage (Table 2).
Table 3 shows the variables that remained in the multivariate analysis and defined an explanatory model for viral infection. The GSTT1 null allele was defined as a protective factor (ORadj 0.219; 95% CI 0.078–0.618; p < 0.004), that is, women who have GSTT1 deletion are less susceptible to HPV infection than women in the control group. On the other hand, smoking was recognized as a risk factor, increasing the chance of viral infection up to 3.6 times (ORadj 3.678; 95% CI 1.111–12.171; p < 0.033). No interaction was observed between variables.
Discussion
To the best of our knowledge, this study’s results have shown, for the first time, that the GSTT1 null allele was characterized as a protective factor against viral infection, that is, women with GSTT1 deletion are less susceptible to HPV infection than women in the control group. On the other hand, smoking was defined as a risk factor, increasing the chance of viral infection up to 3.6 times.
Overall, individuals with GSTM1 and GSTT1 null genotypes are mostly susceptible to damage caused by oxidative stress [27]. This characteristic limits the combination of metabolites during phase II of xenobiotic metabolism, increasing the damage caused by oxidative stress and facilitating infection and viral replication [17]. This genotypic profile could also affect the natural history of infection of HPV STI [13].
Various pathologies, including rheumatoid arthritis, age-related macular degeneration, prostate cancer, lung cancer, CC, and schizophrenia have reported GSTM1 and GSTT1 SNPs [28,29,30]. Moreover, a recent study suggests that patients with severe acute respiratory syndrome (SARS) caused by SARS-CoV-2 (COVID-19) and with GSTT1 null genotype had higher mortality than those with the active allele [31]. More than 90% of CC cases are associated with HPV and several studies suggest that the GST null allele is associated with cancer development; however, these data differ between populations [20]. Most studies therefore prioritize the relationship between the deleted allele with the general course of the disease and not with the susceptibility of infection [17, 22].
Several studies suggest that individuals with homozygous deletion of both genes have limited or insufficient production of enzymes, decreasing detoxification and increasing the malignant transformation of cells which promote carcinogenesis [32]. Cancers related to the presence of the virus are head and neck cancer [33] and CC [19].
This study found no relationship between GSTM1 polymorphism and viral infection. However, it showed for the first time that the deleted GSTT1 decreases chances of HPV infection, protecting women with the null genotype (GSTT1−). Research conducted in patients with HIV suggests that deletions in GSTM1 and GSTT1 genes can slow disease progression [18]. Iorio et al. [34] confirm that GSTT1− is a protective genetic marker for allergic rhinitis. Another study found that both the GSTT1 deleted allele and its combined form with the GSTM1 null are protective factors for schizophrenia and reduce the Chinese population’s susceptibility to it [29].
Chen and Nirunsuksiri [35] observed that HPV infection behaves differently according to GST genotypes. Other studies believe that the product of GSTT1 is protects the host’s DNA against mutations [36]. Foppoli et al. [37] reported that high-risk subtypes can modulate and counteract the effects of increased levels of ROS (reactive oxygen species) in infected cells, that is, viral oncoproteins allow infected cells to survive in a hostile oxidizing environment, preventing the oxidation of anti-apoptotic and detoxifying enzymes. The E6 and E7 genes modulate cellular microRNAs that regulate genes associated with antioxidant response, suppression of OS-induced apoptosis, and regulation of antioxidant enzymes and compounds [38].
The activity of several antioxidant proteins, including those from the catalase family, peroxiredoxins, quinone oxidoreductase 1, and superoxide dismutase (SOD), can be disrupted by viral infections [38]. Furthermore, the expression of oncoproteins is associated with high levels of detoxifying enzymes, such as GSTs and GSH, giving the host cell an escape system from oxidative damage and resisting programmed cell death [38].
Similarly, Lee et al. [22] found that the GSTT1 null allele was protective in women infected with high-risk HPV even with the development of CC. However, the authors did not assess its association with susceptibility to infection. A recent systematic review suggested a risk association between null GSTT1 and infection by high-risk HPV subtypes [23]. Several studies confirm that GSTM1 and GSTT1 null alleles likely affect the progression of several types of cancer, including CC [19, 28], but this finding is still divergent. More studies on the association of polymorphisms with infection by HPV and other viral agents are essential to better understand results similar to ours.
Different than GST polymorphism, smoking was identified as a risk factor for viral infection. In this study, the risk of HPV infection in smokers and ex-smokers was 3.6 times higher than in those who had never smoked. A Finnish study revealed that female smokers had a 1.76-fold higher susceptibility to infection, especially of subtype HPV-16 [39]. Another study, conducted in Greece, showed that women who smoke are 1.7 times more likely to be infected [40]. Several studies indicate tobacco smoking as a risk factor for HPV in different populations [41]. Tobacco smoking affects the immune system and increases the risk of new virus infections and HPV persistence [42]. Simen-Kapeu et al. [42] reported that young female smokers have a limited production of antibodies to fight high-risk oncogenic subtypes. Bergqvist et al. had similar findings [39]. However, the influence of smoking in the initial phase of HPV infections is still indefinite regarding cervical carcinogenesis [43]. Tobacco metabolites present in uterine cervical mucus decrease the quantity and function of epithelial Langerhans cells. This creates a local immunosuppressive effect which makes the host unable to develop an effective immune response, maximizing the risk of viral and persistent infections, including HPV [44, 45]. Tobacco consumption can also increase the carcinogenic action of the virus by inhibiting INF-γ, TNF-α, and mutations in the p53 gene, preventing apoptosis of the infected cell, and favoring the progression to cervical lesions and cancer [46]. Furthermore, components of cigarettes, including benzopyrene, nicotine, and derivatives, damage the cervical epithelium, favoring viral infection [46].
This study found that smoking characterized the exposure to xenobiotics in women, being a predictive factor for the risk of viral infection. Tian et al. [32] suggested a relationship between GSTT1 deletion and the chance of cervical changes in smokers. However, this and other studies [19] found no interaction between GST polymorphisms and smoking in the investigated population. This study has limitations, including the small number of women with HPV, which interfered with the statistical power of the analyses. Expanding the sample and including other populations to validate the results presented could solve this limitation. However, the case–control study also had strengths and allowed us to conclude that tobacco smoking is an important risk factor for viral infection. Furthermore, this was the first study to effectively assess the relationship between GSTM1 and GSTT1 and HPV STI, showing that the GSTT1 null allele can protect women from infection.
Conclusion
This study was conducted in the Municipality of Francisco Beltrão, in the State of Paraná. Results were unprecedented and characterized the GSTT1 null allele as a protective factor against viral infection, showing that women who have GSTT1 deletion are less susceptible to HPV infection than women in the control group. Moreover, smoking was defined as a risk factor, increasing the chance for viral infection in up to 3.6 times. Finally, other studies could expand the sample, dividing the groups between GSTT1−/GSTT1+ genotypes and smokers and non-smokers to see if they are associated.
Consent to publish
All authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
Tumban E (2019) A current update on human papillomavirus-associated head and neck cancers. Viruses 11(10):922–941. https://doi.org/10.3390/v11100922
Coser J, da Boeira TR, Wolf JM, Cerbaro K, Simon D, Lunge VR (2016) Cervical human papillomavirus infection and persistence: a clinic-based study in the countryside from South Brazil. Braz J Infect Dis 20(1):61–68. https://doi.org/10.1016/j.bjid.2015.10.008
Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB (2018) Trends in human papillomavirus-associated cancers—United States, 1999–2015. Morb Mortal Wkly Rep 67(33):918–924. https://doi.org/10.15585/mmwr.mm6733a2
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S (2007) Human papillomavirus and cervical cancer. Lancet 370(9590):890–907. https://doi.org/10.1016/S0140-6736(07)61416-0
Wendland EM, Villa LL, Unger ER, Domingues CM, Benzaken AS, POP-Brazil Study Group (2020) Prevalence of HPV infection among sexually active adolescents and young adults in Brazil: the POP-Brazil Study. Sci Rep 10(1):1–10. https://doi.org/10.1038/s41598-020-61582-2
Melo A, Montenegro S, Liempi S, Moreno S, de La Barra T, Guzmán P, Bustos L, Fonseca-Salamanca F (2019) Frequency of cervical cytological alterations and human papilloma virus in a sample of university students in Temuco, Chile. Rev Chil Infectol 36(4):421–427. https://doi.org/10.4067/S0716-10182019000400421
Iversen L, Fielding S, Lidegaard Ø, Hannaford PC (2021) Contemporary hormonal contraception and cervical cancer in women of reproductive age. Int J Cancer 149(4):769–777. https://doi.org/10.1002/ijc.33585
Siokos AG, Siokou-Siova O, Tzafetas I (2019) Correlation between cervical carcinogenesis and tobacco use by sexual partners. Hell J Nucl Med 22(Suppl 2):184–190. https://pubmed.ncbi.nlm.nih.gov/31802062/. Accessed 16 Sep 2020
Stanley MA (2012) Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev 25(2):215–222. https://doi.org/10.1128/CMR.05028-11
Cruz-Gregorio A, Manzo-Merino J, Lizano M (2018) Cellular redox, cancer and human papillomavirus. Virus Res 246:35–45. https://doi.org/10.1016/j.virusres.2018.01.003
Pudney J, Quayle AJ, Anderson DJ (2005) Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone1. Biol Reprod 73(6):1253–1263. https://doi.org/10.1095/biolreprod.105.043133
Hollman AL, Tchounwou PB, Huang HC (2016) The association between gene-environment interactions and diseases involving the human GST superfamily with SNP variants. Int J Environ Res Public Health 13(4):379–392. https://doi.org/10.3390/ijerph13040379
Tew KD, Manevich Y, Grek C, Xiong Y, Uys J, Townsend DM (2011) The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med 51(2):299–313. https://doi.org/10.1016/j.freeradbiomed.2011.04.013
Malik SS, Kazmi Z, Fatima I, Shabbir R, Perveen S, Masood N (2016) Genetic polymorphism of GSTM1 and GSTT1 and risk of prostatic carcinoma—a meta-analysis of 7,281 prostate cancer cases and 9,082 healthy controls. Asian Pac J Cancer Prev 17(5):2629–2635. https://doi.org/10.7314/APJCP.2016.17.5.2629
Pacholak LM, Kern R, de Oliveira ST, Lúcio LC, Amarante MK, Guembarovski RL, Watanabe M, Panis C (2021) Effects of GSTT1 and GSTM1 polymorphisms in glutathione levels and breast cancer development in Brazilian patients. Mol Biol Rep 48(1):33–40. https://doi.org/10.1007/s11033-020-06107-w
Sudenga SL, Shrestha S, Macaluso M, Partridge EE, Johanning GL, Piyathilake CJ (2014) Functional variants in CYP1A1 and GSTM1 are associated with clearance of cervical HPV infection. Gynecol Oncol 135(3):560–564. https://doi.org/10.1016/j.ygyno.2014.09.015
Kuleape JA, Tagoe EA, Puplampu P, Bonney EY, Quaye O (2018) Homozygous deletion of both GSTM1 and GSTT1 genes is associated with higher CD4+ T cell counts in Ghanaian HIV patients. PLoS ONE 13(5):e0195954. https://doi.org/10.1371/journal.pone.0195954
Gao LB, Pan XM, Li LJ, Liang WB, Bai P, Rao L, Su XW, Wang T, Zhou B, Wei YG, Zhang L (2011) Null genotypes of GSTM1 and GSTT1 contribute to risk of cervical neoplasia: an evidence-based meta-analysis. PLoS ONE 6(5):e20157. https://doi.org/10.1371/journal.pone.0020157
Stosic I, Grujicic D, Arsenijevic S, Brkic M, Milosevic-Djordjevic O (2014) Glutathione S-transferase T1 and M1 polymorphisms and risk of uterine cervical lesions in women from central Serbia. Asian Pac J Cancer Prev 15(7):3201–3205. https://doi.org/10.7314/APJCP.2014.15.7.3201
Joseph T, Chacko P, Wesley R, Jayaprakash PG, James FV, Pillai MR (2006) Germline genetic polymorphisms of CYP1A1, GSTM1 and GSTT1 genes in Indian cervical cancer: associations with tumor progression, age and human papillomavirus infection. Gynecol Oncol 101(3):411–417. https://doi.org/10.1016/j.ygyno.2005.10.033
Lee S-A, Kim JW, Roh JW, Choi JY, Lee KM, Yoo KY, Song YS, Kang D (2004) Genetic polymorphisms of GSTM1, p21, p53 and HPV infection with cervical cancer in Korean women. Gynecol Oncol 93(1):14–18. https://doi.org/10.1016/j.ygyno.2003.11.045
Bortolli APR, Vieira VK, Stefanski EE, Lazarotto AK, Lucio LC (2021) Relationship between GSTM1 and GSTT1 polymorphisms and HPV infection: a systematic review. Mol Biol Rep 48(9):6631–6636. https://doi.org/10.1007/s11033-021-06515-6
Nonnenmacher B, Breitenbach V, Villa LL, Prolla JC, Bozzetti MC (2002) Genital human papillomavirus infection identification by molecular biology among asymptomatic women. Rev Saude Publica 36(1):95–100. https://doi.org/10.1590/S0034-89102002000100015
Trugilo KP, Cebinelli GCM, Berti FCB, Okuyama N, Cezar-Dos-Santos F, Sena MM, Mangieri L, Watanabe M, Oliveira KB (2019) Polymorphisms in the TGFB1 signal peptide influence human papillomavirus infection and development of cervical lesions. Med Microbiol Immunol 208(1):49–58. https://doi.org/10.1007/s00430-018-0557-y
Kiran B, Karkucak M, Ozan H, Yakut T, Ozerkan K, Sag S, Ture M (2010) GST (GSTM1, GSTT1, and GSTP1) polymorphisms in the genetic susceptibility of Turkish patients to cervical cancer. J Gynecol Oncol 21(3):169. https://doi.org/10.3802/jgo.2010.21.3.169
Strange RC, Spiteri MA, Ramachandran S, Fryer AA (2001) Glutathione-S-transferase family of enzymes. Mutat Res Fundam Mol Mech Mutagen 482(1–2):21–26. https://doi.org/10.1016/S0027-5107(01)00206-8
Yadav P, Chatterjee A, Bhattacharjee A (2014) Identification of deleterious nsSNPs in α, μ, π and θ class of GST family and their influence on protein structure. Genomics Data 2:66–72. https://doi.org/10.1016/j.gdata.2014.03.004
Kim SK, Kang SW, Chung JH, Park HJ, Cho KB, Park MS (2015) Genetic polymorphisms of glutathione-related enzymes (GSTM1, GSTT1, and GSTP1) and schizophrenia risk: a meta-analysis. Int J Mol Sci 16(8):19602–19611. https://doi.org/10.3390/ijms160819602
Zhang J, Liu G, Cui X, Yu H, Wang D (2021) Human papillomavirus genotypes and the risk factors associated with multicentric intraepithelial lesions of the lower genital tract: a retrospective study. BMC Infect Dis 21(1):1–9. https://doi.org/10.1186/s12879-021-06234-0
Abbas M, Verma S, Verma S, Siddiqui S, Khan FH, Raza ST, Siddiqi Z, Eba A, Mahdi F (2021) Association of GSTM1 and GSTT1 gene polymorphisms with COVID-19 susceptibility and its outcome. J Med Virol 93(9):5446–5451. https://doi.org/10.1002/jmv.27076
Tian S, Yang X, Zhang L, Zhao J, Pei M, Yu Y, Yang T (2019) Polymorphic variants conferring genetic risk to cervical lesions support GSTs as important associated loci. Medicine (US) 98(41):e174-187. https://doi.org/10.1097/MD.0000000000017487
Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T (2018) A review of HPV-related head and neck cancer. J Clin Med 7(9):241. https://doi.org/10.3390/jcm7090241
Iorio A, Polimanti R, Piacentini S, Liumbruno GM, Manfellotto D, Fuciarelli M (2015) Deletion polymorphism of GSTT1 gene as protective marker for allergic rhinitis. Clin Respir J 9(4):481–486. https://doi.org/10.1111/crj.12170
Chen C, Nirunsuksiri W (1999) Decreased expression of glutathione S-transferase M1 in HPV16-transfected human cervical keratinocytes in culture. Carcinogenesis 20(4):699–703. https://doi.org/10.1093/carcin/20.4.699
Rebbeck TR (1997) Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomark Prev 6(9):733–743. http://www.ncbi.nlm.nih.gov/pubmed/9298582. Accessed 16 Sep 2020
Foppoli C, De Marco F, Cini C (1850) Perluigi M (2015) Redox control of viral carcinogenesis: the human papillomavirus paradigm. Biochim Biophys Acta Gen Subj 8:1622–1632. https://doi.org/10.1016/j.bbagen.2014.12.016
Calaf GM, Urzua U, Termini L, Aguayo F (2018) Oxidative stress in female cancers. OncoTarget 9(34):23824–23842. https://doi.org/10.18632/oncotarget.25323
Bergqvist L, Kalliala I, Aro K, Auvinen E, Jakobsson M, Kiviharju M, Virtanen S, Dillner J, Nieminen P, Louvanto K (2021) Distribution of HPV genotypes differs depending on behavioural factors among young women. Microorganisms 9(4):750. https://doi.org/10.3390/microorganisms9040750
Chatzistamatiou K (2013) Smoking and genital human papilloma virus infection in women attending cervical cancer screening in Greece. World J Obstet Gynecol 2(3):53. https://doi.org/10.5317/wjog.v2.i3.53
Kędzierawski P, Huruk-Kuchinka A, Radowicz-Chil A, Mężyk R, Rugała Z, Sadowski J (2021) Human papillomavirus infection predicts a better survival rate in patients with oropharyngeal cancer. Arch Med Sci 17(5):1308–1316. https://doi.org/10.5114/aoms.2019.83658
Simen-Kapeu A, Kataja V, Yliskoski M, Syrjänen K, Dillner J, Koskela P, Paavonen J, Lehtinen M (2008) Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection. Scand J Infect Dis 40(9):745–751. https://doi.org/10.1080/00365540801995360
Eldridge RC, Pawlita M, Wilson L, Castle PE, Waterboer T, Gravitt PE, Schiffman M, Wentzensen N (2017) Smoking and subsequent human papillomavirus infection: a mediation analysis. Ann Epidemiol 27(11):724-730.e1. https://doi.org/10.1016/j.annepidem.2017.10.004
Feng RM, Hu SY, Zhao FH, Zhang R, Zhang X, Wallach AI, Qiao YL (2017) Role of active and passive smoking in high-risk human papillomavirus infection and cervical intraepithelial neoplasia grade 2 or worse. J Gynecol Oncol 28(5):47. https://doi.org/10.3802/jgo.2017.28.e47
Treco IC, Vieira VK, da Silva JC, Treco FR, Ferreto LED, Lucio LC (2021) Prevalência e fatores associados às alterações cervicais em unidades do Sistema Único de Saúde. Rev Gaúcha Enferm 42(e20200233):1–17. https://doi.org/10.1590/1983-1447.2021.20200233
Xie XT, Liu Q, Wu J, Wakui M (2009) Impact of cigarette smoking in type 2 diabetes development. Acta Pharmacol Sin 30(6):784–787. https://doi.org/10.1038/aps.2009.49
Acknowledgements
The Municipal Health Department of Francisco Beltrão/PR which provided access to the Basic Health Units where the collections took place; The Universidade Estadual do Oeste do Paraná that guaranteed access to the Molecular Biology Laboratory where the samples were processed. To the HPV Study Group at the Molecular Biology Laboratory of the Universidade Estadual do Oeste do Paraná, Francisco Beltrão Campus.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: APRB and LCL; Methodology: APRB, VKV, ICT, CRP, GWW, and LCL; Formal analysis and investigation: APRB, GWW, and LCL; Writing—original draft preparation: APRB and LCL; Writing—review and editing: APRB, VKV, and LCL; Resources: ICT and LCL; Supervision: APRB and LCL.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict to interest.
Informed consent
All authors informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bortolli, A.P.R., Vieira, V.K., Treco, I.C. et al. GSTT1 and GSTM1 polymorphisms with human papillomavirus infection in women from southern Brazil: a case–control study. Mol Biol Rep 49, 6467–6474 (2022). https://doi.org/10.1007/s11033-022-07475-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07475-1