Abstract
Background
Heat shock protein 70s (Hsp70s) are major members of the heat shock protein family and play a variety of roles to protect plants against stress. Plant Hsp70s are a conserved and widely expressed family of heat shock proteins. They have two main functional regions: N-terminal nucleic acid binding region and C-terminal substrate binding region.
Methods and results
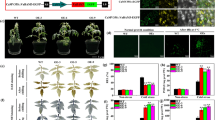
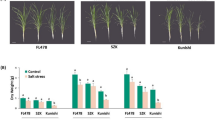
In this study, we cloned the Hsp70 gene of Zostera japonica (ZjHsp70) based on the sequence obtained by transcriptome sequencing. The transcriptional levels of ZjHsp70 increased significantly at 1 h after heat treatment. ZjHsp70 was located in the cytoplasm and nucleus. The overexpression of ZjHsp70 in Arabidopsis resulted in increased heat tolerance, lower contents of malondialdehyde and higher antioxidant enzyme activity than in the wild type. ZjHsp70 may achieve this goal by maintaining highly active antioxidant enzymes.
Conclusions
We show that ZjHsp70 can improve plant heat tolerance by maintaining high antioxidant enzyme activity under high temperature stress. This study provided a basis to study the role of ZjHsp70 in thermotolerance in more detail.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. The nucleotide and deduced amino acid sequence data of ZjHsp70 were registered in GenBank (Accession No. MK359368).
Code availability
Not applicable.
References
Hemminga M, Duarte C (2000) Seagrass ecology. Cambridge University Press, Cambridge
Edgar GJ et al (1994) Comparisons of species richness, size-structure and production of benthos in vegetated and unvegetated habitats in Western Port, Victoria. J Exp Mar Biol Ecol 176(2):201–226
Larkum AWD, Mccomb AJ, Shepherd SA (1991) Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region. Elsevier, Amsterdam
Fan HQ et al (2007) The situations of seagrass resources and researches along Guangxi Coasts of Beibu Gulf. Guangxi Sci 14:289
Short FT, Wyllieecheverria S (1996) Natural and human-induced disturbance of seagrasses. Environ Conserv 23(1):17–27
Durako MJ (1994) Seagrass die-off in Florida Bay (USA): changes in shoot demographic characteristics and population dynamics in Thalassia testudinum. Mar Ecol Progress 110(1):59–66
Janetos AC (1998) Climate change 1995: impacts, adaptations and mitigation of climate change: scientific-technical analyses. Q Rev Biol 73(1):465–477
Lee KS, Sang RP, Kim JB (2005) Production dynamics of the eelgrass, Zostera marina in two bay systems on the south coast of the Korean peninsula. Mar Biol 147(5):1091–1108
Kumar SV, Wigge PA (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140(1):136–147
Jung JH et al (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354(6314):886–889
Liang X, Zhou JM (2018) Receptor-like cytoplasmic kinases: central players in plant receptor kinase-mediated signaling. Annu Rev Plant Biol 69:267–299
Shen H et al (2015) Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat Biotechnol 33(9):996–1003
Chen C et al (2014) A two-locus interaction causes interspecific hybrid weakness in rice. Nat Commun 5:3357
Yu J et al (2017) Two rice receptor-like kinases maintain male fertility under changing temperatures. Proc Natl Acad Sci U S A 114(46):12327–12332
Gao F et al (2012) A heat-activated calcium-permeable channel–Arabidopsis cyclic nucleotide-gated ion channel 6–is involved in heat shock responses. Plant J 70(6):1056–1069
Zhou R et al (2009) Progress in the participation of Ca2+–calmodulin in heat shock signal transduction. Prog Nat Sci 19:1201–1208
Zhang W et al (2009) Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol 149(4):1773–1784
Zhang J et al (2019) Crop Improvement Through Temperature Resilience. Annu Rev Plant Biol 70:753–780
Li XM et al (2015) Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet 47(7):827–833
Zhang SS et al (2017) Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell 29(5):1007–1023
Lee JH, Schöffl F (1996) An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Genet Genomics 252(1):11–19
Kim SR, An G (2013) Rice chloroplast-localized heat shock protein 70, OsHsp70CP1, is essential for chloroplast development under high-temperature conditions. J Plant Physiol 170(9):854–863
Sarkar et al (2013) Functional analysis of Hsp70 superfamily proteins of rice (Oryza sativa). Cell Stress Chaperones 18(4):427–437
Pulido P, Llamas E, Rodriguez-Concepcion M (2017) Both Hsp70 chaperone and Clp protease plastidial systems are required for protection against oxidative stress. Plant Signal Behav 12(3):e1290039
Verma AK et al (2017) Evolutionary conservation and emerging functional diversity of the cytosolic Hsp70: J protein chaperone network of Arabidopsis thaliana. G3 7(6):1941–1954
Thompson JD et al (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876
Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8(3):275–282
Tamura K et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Arnold K et al (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22(2):195–201
Sade N, Galkin E, Moshelion M (2015) Measuring Arabidopsis, tomato and barley leaf relative water content (RWC). Bio Protoc 5(8):e1451
Chen S, Qiu G, Yang M (2019) SMRT sequencing of full-length transcriptome of seagrasses Zostera japonica. Sci Rep 9(1):14537
Jiang J et al (2005) Structural basis of interdomain communication in the Hsc70 chaperone. Mol Cell 20(4):513
Meng G et al (2014) Characterization of CaHsp70-1, a pepper heat-shock protein gene in response to heat stress and some regulation exogenous substances in Capsicum annuum L. Int J Mol Sci 15(11):19741–19759
Cho EK, Choi YJ (2009) A nuclear-localized HSP70 confers thermoprotective activity and drought-stress tolerance on plants. Biotechnol Lett 31(4):597–606
Harm H, Elizabeth C (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11(8):579–592
Lu RC et al (2014) Heat shock protein 70 in Alzheimer’s disease. Biomed Res Int 2014:435203
Zdravko D et al (2014) Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J 25(11):2519–2528
Lin BL et al (2001) Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones 6:201–208
Shinya K et al (2014) Functional analysis of the Hikeshi-like protein and its interaction with HSP70 in Arabidopsis. Biochem Biophys Res Commun 450(1):396–400
Usman MG et al (2017) Molecular analysis of Hsp70 mechanisms in plants and their function in response to stress. Biotechnol Genet Eng Rev 33(1):1
Qi Y et al (2011) Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett 585(1):231–239
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci Cmls 62(6):670–684
Eileen L et al (2009) The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. NeuroImage 45(3):672–678
Sookjin L et al (2009) Heat shock protein cognate 70 – 4 and an E3 ubiquitin ligase, CHIP, mediate plastid-destined precursor degradation through the ubiquitin-26S proteasome system in Arabidopsis. Plant Cell 21(12):3984–4001
Malin A, Morimoto RI, Lea S (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11(8):545–555
Peter T, Denes K (2010) Intrinsically disordered chaperones in plants and animals. Biochem Cell Biol 88(2):167
Grene AR, Neval E, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53(372):1331–1341
Acknowledgements
This study was funded by the Natural Science Foundation of Guangxi Province (2020GXNSFAA297067; 2018GXNSFBA281062), the Research Fund Program of Guangxi Key Lab of Mangrove Conservation and Utilization (GKLMC-21A01; GKLMC-20A04; GKLMC-20A01), the National Natural Science Foundation of China (32170399) and the National Science & Technology Fundamental Resources Investigation Program of China (2019FY100604). These funding bodies had no role in the design of the study, collection, analysis, and interpretation of data, or in the writing of the manuscript.
Funding
This study was funded by the Natural Science Foundation of Guangxi Province (2020GXNSFAA297067; 2018GXNSFBA281062), the Research Fund Program of Guangxi Key Lab of Mangrove Conservation and Utilization (GKLMC-21A01; GKLMC-20A04; GKLMC-20A01), the National Natural Science Foundation of China (32170399) and the National Science & Technology Fundamental Resources Investigation Program of China (2019FY100604). These funding bodies had no role in the design of the study, collection, analysis, and interpretation of data, or in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
SC designed the study and conducted the experiments. GQ conducted field sampling and identification. SC and GQ wrote the manuscript. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, S., Qiu, G. Overexpression of Zostera japonica heat shock protein gene ZjHsp70 enhances the thermotolerance of transgenic Arabidopsis. Mol Biol Rep 49, 6189–6197 (2022). https://doi.org/10.1007/s11033-022-07411-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07411-3