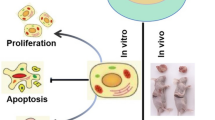
Abstract
Background
Bmal1 and Per2 are the core components of the circadian clock genes (CCGs). Bmal1−/− mice exhibit premature aging, as indicated by hypotrichosis and osteoporosis, with a loss of proliferation ability. The same occurs in Per2−/− mice, albeit to a less severe degree. However, whether the effects of Bmal1 and Per2 on proliferation and osteogenic differentiation are synergistic or antagonistic remains unclear. Thus, our study aimed to explore the effects and specific mechanism.
Methods and results
Lentiviral and adenoviral vectors were constructed to silence or overexpress Bmal1 or Per2 and MTT, flow cytometry, RT-qPCR, WB, immunohistochemistry, alizarin red staining and ChIP-Seq analyses were applied to identify the possible mechanism. The successful knockdown and overexpression of Bmal1/Per2 were detected by fluorescence microcopy. Flow cytometry found out that Bmal1 or Per2 knockdown resulted in G1-phase cell cycle arrest. RT-qPCR showed the different expression levels of Wnt-3a, c-myc1 and axin2 in the Wnt/β-catenin signaling pathway as well as the gene expression change of Rorα and Rev-erbα. Meanwhile, related proteins such as β-catenin, TCF-1, and P-GSK-3β were detected. ALP activity and the amount of mineral nodules were compared. ChIP-Seq results showed the possible mechanism.
Conclusions
Bmal1 and Per2, as primary canonical clock genes, showed synergistic effects on the proliferation and differentiation of BMSCs. They would inhibit the Wnt/β-catenin signaling pathway by downregulating Rorα expression or upregulating Rev-erbα expression, both of which were also key elements of CCGs. And this may be the mechanism by which they negatively regulate the osteogenic differentiation of BMSCs.
Graphical abstract
Bmal1 and Per2 show synergistic effects in the proliferation of BMSCs. In addition, they play a synergistic role in negatively regulating the osteogenic differentiation ability of BMSCs. Bmal1 and Per2 may regulate the aging of BMSCs by altering cell proliferation and osteogenic differentiation through Rorα and Rev-erbα to affect Wnt/β-catenin pathway.








Similar content being viewed by others
Data availability
All datasets generated and/or analyzed during this study are included in this published article.
Abbreviations
- BMSCs:
-
Bone marrow mesenchymal stem
- Bmal1:
-
Brain and muscle arant-like-1
- Per2:
-
Period 2
- Clock:
-
Circadian locomotor output cycles kaput
- Cry:
-
Cryptochrome
- RORα:
-
Retinoic acid-related orphan
- Rev-erb:
-
Retinoid-related orphan receptor α
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription polymerase chain
- RT-qPCR:
-
Real-time fluorescence quantitative
- FBS:
-
Fetal bovine serum
- PBS:
-
Phosphate-buffered saline
- DMSO:
-
Dimethyl sulfoxide
- EDTA:
-
Ethylene diamine tetraacetic acid
- FCM:
-
Flow cytometry
- WB:
-
Western blot
- SA-β-gal:
-
Senescence-associated β-galactosidase
- GFP:
-
Green fluorescent protein
- RFP:
-
Red fluorescent protein
- BSA:
-
Bovine serum albumin
- MTT:
-
Methyl thiazolyl tetrazolium
- CCGs:
-
Circadian clock genes
- ALP:
-
Alkaline phosphatase
- OCN:
-
Osteocalcin
- Runx2:
-
Runt-related transcription factor 2
- Wnt3a:
-
Wingless Int1 3a
- C-myc:
-
Cell-myc1
- TCF1:
-
T-cell-specific transcription factor1
- P-GSK-3:
-
Phosphorylated glycogen synthase kinase-3β
- ChIP-Seq:
-
Chromatin immunoprecipitation and high-throughput sequencing
References
Casado-Díaz A, Dorado G, Giner M, Montoya MJ, Navarro-Valverde C, Díez-Pérez A et al (2019) Proof of concept on functionality improvement of mesenchymal stem-cells, in postmenopausal osteoporotic women treated with teriparatide (PTH1-34), after suffering atypical fractures. Calcif Tissue Int 104:631–640. https://doi.org/10.1007/s00223-019-00533-0
Luo Z, Liu M, Sun L, Rui F (2015) Icariin recovers the osteogenic differentiation and bone formation of bone marrow stromal cells from a rat model of estrogen deficiency-induced osteoporosis. Mol Med Rep 12:382–388. https://doi.org/10.3892/mmr.2015.3369
Qian G, Zhang L, Wang G, Zhao Z, Peng S, Shuai C (2021) 3D printed Zn-doped mesoporous Silica-incorporated poly-L-lactic acid scaffolds for bone repair. Int J Bioprint 7:346. https://doi.org/10.18063/ijb.v7i2.346
Qian G, Teliang Lu, Jing Z, Rui LD et al (2020) Promoting bone regeneration of calcium phosphate cement by addition of PLGA microspheres and zinc silicate via synergistic effect of in-situ pore generation, bioactive ion stimulation and macrophage immunomodulation. Appl Mater Today. https://doi.org/10.1016/j.apmt.2020.100615
Kondratov VR (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20:1868–1873. https://doi.org/10.1101/gad.1432206
Mcdearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL et al (2006) Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314:1304–1308. https://doi.org/10.1126/science.1132430
Hood S, Amir S (2017) The aging clock: circadian rhythms and later life. J Clin Investig 127:437–446. https://doi.org/10.1172/JCI90328
Onoue T, Nishi G, Hikima J, Sakai M, Kono T (2019) Circadian oscillation of TNF-alpha gene expression regulated by clock gene, BMAL1 and CLOCK1, in the Japanese medaka (Oryzias latipes). Int Immunopharmacol 70:362–371. https://doi.org/10.1016/j.intimp.2019.02.004
Burish MJ, Chen Z, Yoo SH (2019) Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiol (Oxf) 225:e13161. https://doi.org/10.1111/apha.13161
Framnes-DeBoer SN, Bakke E, Yalamanchili S, Peterson H, Sandoval DA, Seeley RJ et al (2020) Bromocriptine improves glucose tolerance independent of circadian timing, prolactin, or the melanocortin-4 receptor. Am J Physiol Endocrinol Metab 318:E62-e71. https://doi.org/10.1152/ajpendo.00325.2019
Lin F, Chen Y, Li X, Zhao Q, Tan Z (2013) Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem Funct 31:166–172. https://doi.org/10.1002/cbf.2871
Tsang K, Liu H, Yang Y, Charles JF, Ermann J (2019) Defective circadian control in mesenchymal cells reduces adult bone mass in mice by promoting osteoclast function. Bone 121:172–180. https://doi.org/10.1016/j.bone.2019.01.016
Takarada T, Xu C, Ochi H, Nakazato R, Yamada D, Nakamura S et al (2017) Bone resorption is regulated by circadian clock in osteoblasts. J Bone Miner Res 32:872–881. https://doi.org/10.1002/jbmr.3053
Murayama Y, Yahagi N, Takeuchi Y, Aita Y, Mehrazad Saber Z, Wada N et al (2019) Glucocorticoid receptor suppresses gene expression of Rev-erbα (Nr1d1) through interaction with the CLOCK complex. FEBS Lett 593:423–432. https://doi.org/10.1002/1873-3468.13328
Yiew NKH, Chatterjee TK, Tang YL, Pellenberg R, Stansfield BK, Bagi Z et al (2017) A novel role for the Wnt inhibitor APCDD1 in adipocyte differentiation: implications for diet-induced obesity. J Biol Chem 292:6312–6324. https://doi.org/10.1074/jbc.M116.758078
Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WEB (2009) Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 45:726–735. https://doi.org/10.1016/j.bone.2009.06.015
Tamaru T, Takamatsu K (2018) Circadian modification network of a core clock driver BMAL1 to harmonize physiology from brain to peripheral tissues. Neurochem Int 119:11–16. https://doi.org/10.1016/j.neuint.2017.12.013
Flores-Hernández E, Velázquez DM, Castañeda-Patlán MC, Fuentes-García G, Fonseca-Camarillo G, Yamamoto-Furusho JK et al (2020) Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells. Cell Signal 72:109636. https://doi.org/10.1016/j.cellsig.2020.109636
Shimozaki S, Yamamoto N, Domoto T, Nishida H, Hayashi K, Kimura H et al (2016) Efficacy of glycogen synthase kinase-3 beta targeting against osteosarcoma via activation of beta-catenin. Oncotarget 7:77038–77051. https://doi.org/10.18632/oncotarget.12781
Zhuo H, Wang Y, Zhao Q (2018) The interaction between Bmal1 and Per2 in mouse BMSC osteogenic differentiation. Stem Cells Int 2018:3407821. https://doi.org/10.1155/2018/3407821
Doumpas N, Lampart F, Robinson MD, Lentini A, Nestor CE, Cantù C et al (2019) TCF/LEF dependent and independent transcriptional regulation of Wnt/β-catenin target genes. Embo J. https://doi.org/10.15252/embj.201798873
Boucher H, Vanneaux V, Domet T, Parouchev A, Larghero J (2016) Circadian clock genes modulate human bone marrow mesenchymal stem cell differentiation, migration and cell cycle. PLoS ONE 11:e0146674. https://doi.org/10.1371/journal.pone.0146674
He Y, Lin F, Chen Y, Tan Z, Bai D, Zhao Q (2015) Overexpression of the circadian clock gene rev-erbα affects murine bone mesenchymal stem cell proliferation and osteogenesis. Stem Cells Dev 24:1194–1204. https://doi.org/10.1089/scd.2014.0437
Eckstein A, Grzyb J, Hermanowicz P, Łabuz J, Banaś AK (2019) A role for GLABRA1 in dark-induced senescence. Acta Biochim Pol 66:243–248. https://doi.org/10.18388/abp.2018_2825
Rolph DN, Deb M, Kanji S, Greene CJ, Das M, Joseph M et al (2020) Ferutinin directs dental pulp-derived stem cells towards the osteogenic lineage by epigenetically regulating canonical Wnt signaling. Biochim Biophys Acta Mol Basis Dis 1866:165314. https://doi.org/10.1016/j.bbadis.2018.10.032
Ge X, Shi R, Ma X (2017) The secreted protein WNT5A regulates condylar chondrocyte proliferation, hypertrophy and migration. Arch Oral Biol 82:171–179. https://doi.org/10.1016/j.archoralbio.2017.06.019
Zou Y, Salinas P (2014) Introduction: Wnt signaling mechanisms in development and disease. Dev Neurobiol 74:757–758. https://doi.org/10.1002/dneu.22192
Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W (2007) Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA 104:18531–18536. https://doi.org/10.1073/pnas.0703113104
Shimozaki S, Yamamoto N, Domoto T, Nishida H, Hayashi K, Kimura H et al (2016) Efficacy of glycogen synthase kinase-3β targeting against osteosarcoma via activation of β-catenin. Oncotarget 7:77038–77051. https://doi.org/10.18632/oncotarget.12781
Kriz V, Korinek V (2018) Wnt, RSPO and Hippo signalling in the intestine and intestinal stem cells. Genes (Basel) 9:20. https://doi.org/10.3390/genes9010020
Kim M, Jho EH (2014) Cross-talk between Wnt/β-catenin and Hippo signaling pathways: a brief review. BMB Rep 47:540–545. https://doi.org/10.5483/bmbrep.2014.47.10.177
Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S et al (2014) YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158:157–170. https://doi.org/10.1016/j.cell.2014.06.013
Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW et al (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162:780–794. https://doi.org/10.1016/j.cell.2015.07.013
Chen J, Long F (2018) mTOR signaling in skeletal development and disease. Bone Res 6:1. https://doi.org/10.1038/s41413-017-0004-5
Khan M, Muzumdar D, Shiras A (2019) Attenuation of tumor suppressive function of FBXO16 ubiquitin ligase activates Wnt signaling in glioblastoma. Neoplasia 21:106–116. https://doi.org/10.1016/j.neo.2018.11.005
Jager J, Wang F, Fang B, Lim HW, Peed LC, Steger DJ et al (2016) The nuclear receptor rev-erbα regulates adipose tissue-specific FGF21 signaling. J Biol Chem 291:10867–10875. https://doi.org/10.1074/jbc.M116.719120
Shin D, Kim IS, Lee JM, Shin SY, Lee JH, Baek SH et al (2014) The hidden switches underlying RORα-mediated circuits that critically regulate uncontrolled cell proliferation. J Mol Cell Biol 6:338–348. https://doi.org/10.1093/jmcb/mju023
Uriz-Huarte A, Date A, Ang H, Ali S, Brady HJM, Fuchter MJ (2020) The transcriptional repressor REV-ERB as a novel target for disease. Bioorg Med Chem Lett 30:127395. https://doi.org/10.1016/j.bmcl.2020.127395
Min HY, Son HE, Jang WG (2019) Estradiol-induced RORα expression positively regulates osteoblast differentiation. Steroids 149:108412. https://doi.org/10.1016/j.steroids.2019.05.004
Salehi M, Kamali E, Karahmadi M, Mousavi SM (2017) RORA and autism in The Isfahan population: is there an epigenetic relationship. Cell J 18:540–546. https://doi.org/10.22074/cellj.2016.4720
Green AC, Martin TJ, Purton LE (2016) The role of vitamin A and retinoic acid receptor signaling in post-natal maintenance of bone. J Steroid Biochem Mol Biol 155:135–146. https://doi.org/10.1016/j.jsbmb.2015.09.036
Park JS, Moon SJ, Lim MA, Byun JK, Hwang SH, Yang S et al (2019) Retinoic acid receptor-related receptor alpha ameliorates autoimmune arthritis via inhibiting of Th17 cells and osteoclastogenesis. Front Immunol 10:2270. https://doi.org/10.3389/fimmu.2019.02270
Meyer T, Kneissel M, Mariani J, Fournier B (2000) In vitro and in vivo evidence for orphan nuclear receptor RORalpha function in bone metabolism. Proc Natl Acad Sci USA 97:9197–9202. https://doi.org/10.1073/pnas.150246097
Yang N, Meng QJ (2016) Circadian clocks in articular cartilage and bone: a compass in the sea of matrices. J Biol Rhythms 31:415–427. https://doi.org/10.1177/0748730416662748
Park SC, Park IG, Kim H, Lee JM (2019) N-terminal domain mediated regulation of RORα1 inhibits invasive growth in prostate cancer. Int J Mol Sci 20:1684. https://doi.org/10.3390/ijms20071684
Chatterjee S, Ma K (2016) Circadian clock regulation of skeletal muscle growth and repair. F1000Research 5:1549. https://doi.org/10.12688/f1000research.9076.1
Kim K, Kim JH, Kim I, Seong S, Kim N (2020) Rev-erbα Negatively regulates osteoclast and osteoblast differentiation through p38 MAPK signaling pathway. Mol Cells 43:34–47. https://doi.org/10.14348/molcells.2019.0232
Funding
This work was supported by the National Science Foundation of China (No. 81870745 and No. 81371113).
Author information
Authors and Affiliations
Contributions
JZ and LZ designed the projects, acquired the data and wrote the manuscript. QZ and ZT designed the projects and approved the draft. XW and YY constructed the figures and approved the draft. RL interpreted the data and approved the draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
The Research Ethics Committee of State Key Laboratory of Oral Diseases reviewed our research project and made the resolution as the following: The experiment involved ethical part conforms to the scientific experiment ethical requirement, it is agreed to implement the experiment.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, J., Zhang, L., Tan, Z. et al. Bmal1- and Per2-mediated regulation of the osteogenic differentiation and proliferation of mouse bone marrow mesenchymal stem cells by modulating the Wnt/β-catenin pathway. Mol Biol Rep 49, 4485–4501 (2022). https://doi.org/10.1007/s11033-022-07292-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07292-6