Abstract
Background
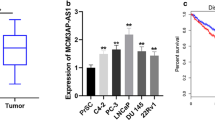
Prostate cancer antigen 3 (PCA3) is the most promising diagnostic biomarker for the differential diagnosis of prostate cancer identified to date. As a dominant-negative oncogene, PCA3 negatively regulates the expression of tumor suppressor PRUNE2 (a human homolog of the Drosophila prune gene) gene. Although interaction between PCA3-PRUNE2 was clearly reported, the precise mechanism how PCA3 is upregulated in prostate cancer remained highly elusive. Accordingly, here we aimed demonstrate the role of microRNAs in PCA3 upregulation and interplay between these miRNAs and PCA3-PRUNE2 axis.
Methods and results
We evaluated expression of PCA3, PRUNE2 and miRNAs by quantitative reverse transcription polymerase chain reaction. Overexpression and silencing of miRNAs were achieved by synthetic miRNA mimics and inhibitors, respectively. Colony formation, migration, apoptosis, and cell cycle assays were performed to reveal the effects of miRNA modulation. We identified that PCA3 expression was significantly downregulated in both prostate cancer tissues and cells and inversely correlated with the expressions of miR-19a and miR-421. Restoring the functions of miR-19a and miR-421 by miRNA mimics significantly downregulated the expression of PCA3 and promoted apoptosis and cell cycle blockade and interfered with the proliferation and migration in prostate cancer cells. Conversely, silencing the expressions of these miRNAs yielded the opposite effect.
Conclusions
Collectively, our results uncover a previously unrecognized novel mechanism on PCA3 upregulation in prostate cancer and proved that miR-19a and miR-421 might be responsible for the increased expression of PCA3, indicating that both miRNAs might be novel candidates for prostate cancer diagnosis and therapy.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Harris JL, Richards RS, Chow CW, Lee S, Kim M, Buck M et al (2013) BMCC1 is an AP-2 associated endosomal protein in prostate cancer cells. PLoS ONE 8(9):e73880
Roobol MJ, Kerkhof M, Schröder FH, Cuzick J, Sasieni P, Hakama M et al (2009) Prostate cancer mortality reduction by prostate-specific antigen–based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur Urol 56(4):584–591
Bussemakers MJ, Van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA et al (1999) DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res 59(23):5975–5979
Hessels D, Schalken JA (2009) The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol 6(5):255
Wolf AM, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ et al (2010) American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin 60(2):70–98
Chunhua L, Zhao H, Zhao H, Lu Y, Wu J, Gao Z et al (2018) Clinical significance of peripheral blood PCA3 gene expression in early diagnosis of prostate cancer. Transl Oncol 11(3):628–632
Salameh A, Lee AK, Cardó-Vila M, Nunes DN, Efstathiou E, Staquicini FI et al (2015) PRUNE2 is a human prostate cancer suppressor regulated by the intronic long noncoding RNA PCA3. Proc Natl Acad Sci 112(27):8403–8408
Teixeira AA, Marchiò S, Dias-Neto E, Nunes DN, da Silva IT, Chackerian B et al (2017) Going viral? Linking the etiology of human prostate cancer to the PCA 3 long noncoding RNA and oncogenic viruses. EMBO Mol Med 9(10):1327–1330
Rennie W, Liu C, Carmack CS, Wolenc A, Kanoria S, Lu J et al (2014) STarMir: a web server for prediction of microRNA binding sites. Nucleic Acids Res 42(W1):W114–W118
Anastasiadou E, Jacob LS, Slack FJ (2018) Non-coding RNA networks in cancer. Nat Rev Cancer 18(1):5
Walsh AL, Tuzova AV, Bolton EM, Lynch TH, Perry AS (2014) Long noncoding RNAs and prostate carcinogenesis: the missing ‘linc’? Trends Mol Med 20(8):428–436
Gezer U, Tiryakioglu D, Bilgin E, Dalay N, Holdenrieder S (2015) Androgen stimulation of PCA3 and miR-141 and their release from prostate cancer cells. Cell J (Yakhteh) 16(4):488
Zhang G, He X, Ren C, Lin J, Wang Q (2019) Long noncoding RNA PCA3 regulates prostate cancer through sponging miR-218-5p and modulating high mobility group box 1. J Cell Physiol 234(8):13097–13109. https://doi.org/10.1002/jcp.27980
He JH, Li BX, Han ZP, Zou MX, Wang L, Lv YB et al (2016) Snail-activated long non-coding RNA PCA3 up-regulates PRKD3 expression by miR-1261 sponging, thereby promotes invasion and migration of prostate cancer cells. Tumour Biol. https://doi.org/10.1007/s13277-016-5450-y
Wa Q, Li L, Lin H, Peng X, Ren D, Huang Y et al (2018) Downregulation of miR-19a-3p promotes invasion, migration and bone metastasis via activating TGF-β signaling in prostate cancer. Oncol Rep 39(1):81–90. https://doi.org/10.3892/or.2017.6096
Feng S, Zhu X, Fan B, Xie D, Li T, Zhang X (2016) miR-19a-3p targets PMEPA1 and induces prostate cancer cell proliferation, migration and invasion. Mol Med Rep 13(5):4030–4038
Acknowledgements
This study complies with the publication guidelines of TCGA, and the results presented here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Funding
The present study was supported by the Grant from the Adiyaman University (Grand Number: SHMYOMAP/2020–0001).
Author information
Authors and Affiliations
Contributions
EB: Conceptualization, Methodology, Data curation, Writing—original draft, Writing—review & editing SK: Conceptualization, Methodology, Writing—review & editing ET: Methodology, Writing—review & editing HB: Methodology, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
There is no potential source of conflict of interest to report.
Ethical approval
Not required.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bozgeyik, E., Kocahan, S., Temiz, E. et al. miR-19a and miR-421 target PCA3 long non-coding RNA and restore PRUNE2 tumor suppressor activity in prostate cancer. Mol Biol Rep 49, 6803–6815 (2022). https://doi.org/10.1007/s11033-021-06996-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06996-5