Abstract
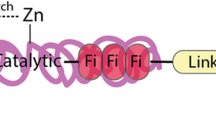
Matrix metalloproteinases (MMPs) or matrixins, are members of a zinc-dependent endopeptidase family. They cause remodeling of the extracellular matrix (ECM) leading to numerous diseases. MMPs subfamilies possess: collagenases, gelatinases, stromelysins and membrane-type MMPs (MT-MMP). They consist of several domains; pro-peptide, catalytic, linker peptide and the hemopexin (Hpx) domains. MMPs are involved in initiation, proliferation and metastasis of cancer through the breakdown of ECM physical barriers. Overexpression of MMPs is associated with poor prognosis of cancer. This review will discuss both types of MMPs and current inhibitors, which target them in different aspects, including, biosynthesis, activation, secretion and catalytic activity. Several synthetic and natural inhibitors of MMPs (MMPIs) that can bind the catalytic domain of MMPs have been designed including; peptidomimetic, non-peptidomimetic, tetracycline derivatives, off-target MMPI, natural products, microRNAs and monoclonal antibodies.
Graphic Abstract


Similar content being viewed by others
Data availability
Data were obtained from cancer registries and published information. The journal after publication is authorized to make all data available.
Abbreviations
- ADAMs:
-
A disintegrin and metalloproteinase
- ADAMTs:
-
A disintegrin and metalloproteinases with thrombospondin motifs
- AKT:
-
Serine/threonine-specific protein kinase
- AP-1:
-
Activator protein-1
- BAE:
-
Bovine aortic endothelial
- ECM:
-
Extracellular matrix
- EGCG:
-
Epigallocatechin—Gallate
- EGF:
-
Epidermal growth factor
- EMT:
-
Epithelial-to-mesenchymal transition
- ERK:
-
Extracellular signal–regulated kinase
- Fab:
-
Antibody fragment
- FAK:
-
Focal adhesion kinase
- HIF-1:
-
Hypoxia-inducible factor 1
- Hpx:
-
Hemopexin
- MAPK:
-
Mitogen-activated protein kinase
- MHC:
-
Major histocompatibility complex
- mAbs:
-
Monoclonal antibodies
- MMPs:
-
Matrix metalloproteinases
- MMPIs:
-
MMP inhibitors
- MT-MMP:
-
Membrane-type MMPs
- mTOR:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor κB
- siRNA:
-
Small interfering RNA
- TACE:
-
TNF-α converting enzyme
- TGF-β:
-
Transforming growth factor-β
- TIMPs:
-
Tissue inhibitors of metalloproteinases
- uPA:
-
Urokinase-type plasminogen activator
- uPAR:
-
Urokinase-type plasminogen activator receptor
References
Kumar GB, Nair BG, Perry JJP, Martin DB (2019) Recent insights into natural product inhibitors of matrix metalloproteinases. MedChemComm 10:2024–2037
Lenci E, Cosottini L, Trabocchi A (2021) Novel matrix metalloproteinase inhibitors: an updated patent review (2014–2020). Expert Opin Ther Patents 31:509–523
Wen D, Chen Z, Zhang Z, Jia Q (2020) The expression, purification, and substrate analysis of matrix metalloproteinases in Drosophila melanogaster. Protein Exp Purif 171:105629
Kapoor C, Vaidya S, Wadhwan V, Kaur G, Pathak A (2016) Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther 12:28
Khokha R, Murthy A, Weiss A (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13:649–665
Nguyen TT, Ding D, Wolter WR, Pérez RL, Champion MM, Mahasenan KV, Hesek D, Lee M, Schroeder VA, Jones JI, Lastochkin E, Rose MK, Peterson CE, Suckow MA, Mobashery S, Chang M (2018) Validation of matrix metalloproteinase-9 (MMP-9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small-molecule MMP-9 inhibitor that accelerates healing. J Med Chem 61:8825–8837
Winer A, Adams S, Mignatti P (2018) Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther 17:1147–1155
Lian G-Y, Wang Q-M, Mak TS-K, Huang X-R, Yu X-Q, Lan H-Y (2021) Inhibition of tumor invasion and metastasis by targeting TGF-β-Smad-MMP2 pathway with Asiatic acid and Naringenin. Mol Ther 20:277–289
Puente XS, Sánchez LM, Overall CM, López-Otín C (2003) Human and mouse proteases: a comparative genomic approach. Nat Rev Genet 4:544–558
Dufour A, Overall CM (2013) Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci 34:233–242
Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari NV, Monjezi MR, Ilbeigi S, Alahari SK (2021) Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene 40:1043–1063
Zhong Y, Lu Y-T, Sun Y, Shi Z-H, Li N-G, Tang Y-P, Duan J-A (2018) Recent opportunities in matrix metalloproteinase inhibitor drug design for cancer. Expert Opin Drug Discov 13:75–87
Klein T, Bischoff R (2010) Active metalloproteases of the A Disintegrin and Metalloprotease (ADAM) family: biological function and structure. J Proteome Res 10:17–33
Xu I, Thériault M, Brunette I, Rochette PJ, Proulx S (2021) Matrix metalloproteinases and their inhibitors in Fuchs endothelial corneal dystrophy. Exp Eye Res 205:108500
Javaid MA, Abdallah M-N, Ahmed AS, Sheikh Z (2013) Matrix metalloproteinases and their pathological upregulation in multiple sclerosis: an overview. Acta Neurol Belg 113:381–390
Fischer T, Riedl R (2021) Challenges with matrix metalloproteinase inhibition and future drug discovery avenues. Expert Opin Drug Discov 16:75–88
Bode W, Gomis-Rüth F-X, Stöckler W (1993) Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins.’ FEBS Lett 331:134–140
Wolak D, Sechman A, Hrabia A (2021) Effect of eCG treatment on gene expression of selected matrix metalloproteinases (MMP-2, MMP-7, MMP-9, MMP-10, and MMP-13) and the tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary. Anim Reprod Sci 224:106666
Makowski GS, Ramsby ML (1998) Binding of matrix metalloproteinase 9 to fibrin is mediated by amorphous calcium-phosphate. Inflammation 22:599–617
Cui N, Hu M, Khalil RA (2017) Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 147:1–73
Öztürk VÖ, Meriç P, Sorsa T, Tervahartiala T, Bostanci N, Nwhator SO, Emingil G (2021) Regulation of matrix metalloproteinases-8,-9 and endogenous tissue inhibitor-1 in oral biofluids during pregnancy and postpartum. Arch Oral Biol 124:105065
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69:562–573
Okada Y, Morodomi T, Enghild JJ, Suzuki K, Yasui A, Nakanishi I, Salvesen G, Nagase H (1990) Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem 194:721–730
Mannello F, Tonti G, Papa S (2005) Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr Cancer Drug Targets 5:285–298
Noël A, Jost M, Maquoi E (2008) Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol 19:52–60
Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P (2005) ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA 102:9182–9187
Krzyzanowska-Gołab D, Lemańska-Perek A, Katnik-Prastowska I (2007) Fibronectin as an active component of the extracellular matrix. Postepy Hig Med Dosw(Online) 61:655–663
Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R (2011) Interaction of tumor cells with the microenvironment. Cell Commun Signal 9:18–18
Lu YE, Chen YJ (2021) Resveratrol inhibits matrix metalloproteinase-1 and-3 expression by suppressing of p300/NFκB acetylation in TNF-α-treated human dermal fibroblasts. Chem-Biol Interact 337:109395
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochem Biophys Acta 1833:3481–3498
Fouzder C, Mukhuty A, Kundu R (2021) Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch Biochem Biophys 697:108700
Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G (2014) Matrix metalloproteinases and cancer—roles in threat and therapy. Asian Pac J Cancer Prev 15:1085–1091
Deryugina EI, Quigley JP (2010) Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochem Biophys Acta 1803:103–120
Tang M-L, Bai X-J, Li Y, Dai X-J, Yang F (2018) MMP-1 over-expression promotes malignancy and stem-like properties of human osteosarcoma MG-63 cells in vitro. Curr Med Sci 38:809–817
Napoli S, Scuderi C, Gattuso G, Di Bella V, Candido S, Basile MS, Libra M, Falzone L (2020) Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells 9:1151
Geervliet E, Bansal R (2020) Matrix metalloproteinases as potential biomarkers and therapeutic targets in liver diseases. Cells 9:1212
Knapinska AM, Estrada C-A, Fields GB (2017) The roles of matrix metalloproteinases in pancreatic cancer. Prog Mol Biol Transl Sci 148:339–354
Jedryka M, Chrobak A, Chelmonska-Soyta A, Gawron D, Halbersztadt A, Wojnar A, Kornafel J (2012) Matrix metalloproteinase (MMP)-2 and MMP-9 expression in tumor infiltrating CD3 lymphocytes from women with endometrial cancer. Int J Gynecol Cancer 22:1303–1309
Edsparr K, Basse PH, Goldfarb RH, Albertsson P (2011) Matrix metalloproteinases in cytotoxic lymphocytes impact on tumour infiltration and immunomodulation. Cancer Microenviron 4:351–360
Chiou S-H, Sheu B-C, Chang W-C, Huang S-C, Hong-Nerng HJ (2005) Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol 67:35–50
Zhang Y-Y, Chen B, Ding Y-Q (2012) Metastasis-associated factors facilitating the progression of colorectal cancer. Asian Pac J Cancer Prev 13:2437–2444
Shen Z, Wang X, Yu X, Zhang Y, Qin L (2017) MMP16 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Oncotarget 8:72197
Garde A, Sherwood DR (2021) Fueling cell invasion through extracellular matrix. Trends Cell Biol 31:445–456
Scheau C, Badarau IA, Costache R, Caruntu C, Mihai GL, Didilescu AC, Constantin C, Neagu M (2019) The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol 2019:10
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G (2014) Matrix metalloproteinases and cancer: roles in threat and therapy. Asian Pac J Cancer Prev 15:1085–1091
Chen H, He S, Sa G (2021) Podosome formation in the murine palatal mucosae: its proteolytic role in rete peg formation. Ann Anat 235:151703
Choi S, Myers JN (2008) Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res 87:14–32
Birchmeier C, Birchmeier W, Brand-Saberi B (1996) Epithelial-mesenchymal transitions in cancer progression. Acta Anat 156:217–226
Takeichi M (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1455
Han L, Zhou W, Wu F (2021) Long non-coding RNA LOC284454 promotes hepatocellular carcinoma cell invasion and migration by inhibiting E-cadherin expression. J Oncol Rep 45:1–1
Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114:111–118
Illman SA, Lehti K, Keski-Oja J, Lohi J (2006) Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci 119:3856–3865
McCawley LJ, Matrisian LM (2001) Tumor progression: defining the soil round the tumor seed. Curr Biol 11:R25–R27
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
Luo Y, Hu J, Liu Y, Li L, Li Y, Sun B, Kong R (2021) Invadopodia: a potential target for pancreatic cancer therapy. Crit Rev Oncol/Hematol 159:103236
Jin Y-J, Ji Y, Jang Y-P, Choung S-Y (2021) Acer tataricum subsp. ginnala inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and TGFβ/Smad signaling in UVB-irradiated human dermal fibroblasts. Molecules 26:662
Kim J, Yu W, Kovalski K, Ossowski L (1998) Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell 94:353–362
Park JY, Shin M-S (2021) Inhibitory effects of pectic polysaccharide isolated from Diospyros kaki leaves on tumor cell angiogenesis via VEGF and MMP-9 regulation. Polymers 13:64
Gialeli C, Theocharis AD, Karamanos NK (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278:16–27
Gorelik L, Flavell RA (2001) Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 7:1118–1122
Willcockson H, Ozkan H, Chubinskaya S, Loeser RF, Longobardi L (2021) CCL2 induces articular chondrocyte MMP expression through ERK and p38 signaling pathways. Osteoarthr Cartilage Open 3:100136
Piperigkou Z, Manou D, Karamanou K, Theocharis AD (2018) Strategies to target matrix metalloproteinases as therapeutic approach in cancer. In: Cal S, Obaya AJ (eds) Proteases and cancer: methods and protocols. Springer, New York, pp 325–348
Wang XY, Wang YH, Song Z, Hu XY, Wei JP, Zhang J, Wang HS (2021) Recent progress in functional peptides designed for tumor-targeted imaging and therapy. J Mater Chem C 9(11):3749–3772
Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R (2019) Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol 137:57–83
Shi Y, Ma X, Fang G, Tian X, Ge C (2021) Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: recent progress and current challenges. NanoImpact 21:100293
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhibit Med Chem 31:177–183
Kubina R, Iriti M, Kabała-Dzik A (2021) Anticancer potential of selected flavonols: fisetin, kaempferol, and quercetin on head and neck cancers. Nutrients 13:845
Shi Y, Ma X, Fang G, Tian X, Ge C (2021) Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: recent progress and current challenges. NanoImpact. 21:100293
Das N, Benko C, Gill SE, Dufour A (2021) The pharmacological TAILS of matrix metalloproteinases and their inhibitors. Pharmaceuticals 14:31
Li W, Saji S, Sato F, Noda M, Toi M (2013) Potential clinical applications of matrix metalloproteinase inhibitors and their future prospects. Int J Biol Markers 28:117–130
Steward WP, Thomas AL (2000) Marimastat: the clinical development of a matrix metalloproteinase inhibitor. Expert Opin Investig Drugs 9:2913–2922
Yang J-S, Lin C-W, Su S-C, Yang S-F (2016) Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert Opin Drug Metab Toxicol 12:191–200
Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G (2014) Matrix metalloproteinases and cancer-roles in threat and therapy. Asian Pac J Cancer Prev 15:1085–1091
Broccoli A, Zinzani PL (2021) Emerging new small molecules in peripheral T-cell lymphomas. Wiley, New York, pp 343–349
Vihinen P, Kähäri V-M (2002) Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 99:157–166
Hidalgo M, Eckhardt SG (2001) Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 93:178–193
Rudzińska M, Daglioglu C, Savvateeva LV, Kaci FN, Antoine R, Zamyatnin A Jr (2021) Current status and perspectives of protease inhibitors and their combination with nanosized drug delivery systems for targeted cancer therapy. Drug Des Dev Ther 15:9
Kanagaraj AS, Kumar Patel VM (2020) Host modulation therapy: a mini review. Arch Oral Biol 105:72–50
Sapadin AN, Fleischmajer R (2006) Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol 54:258–265
Dedes P, Kanakis I, Gialeli C, Theocharis A, Tsegenidis T, Kletsas D, Tzanakakis G, Karamanos N (2013) Preclinical evaluation of zoledronate using an in vitro mimetic cellular model for breast cancer metastatic bone disease. Biochim Biophys Acta 1830:3625–3634
Li X-Y, Lin Y-C, Huang W-L, Hong C-Q, Chen J-Y, You Y-J, Li W-B (2012) Zoledronic acid inhibits proliferation and impairs migration and invasion through downregulating VEGF and MMPs expression in human nasopharyngeal carcinoma cells. Med Oncol 29:714–720
Coleman R, Cook R, Hirsh V, Major P, Lipton A (2011) Zoledronic acid use in cancer patients: more than just supportive care? Cancer 117:11–23
Moses AS, Demessie AA, Taratula O, Korzun T, Slayden OD, Taratula O (2021) Nanomedicines for endometriosis: lessons learned from cancer research. Small 17(7):2004975
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661
Abdel-Hamid NM, Nazmy MH, Abdel-Bakey AI (2011) Polyol profile as an early diagnostic and prognostic marker in natural product chemoprevention of hepatocellular carcinoma in diabetic rats. Diabetes Res Clin Pract 92:228–237
Elmosallamy A, Abdel-Hamid N, Srour L, Hussein SA (2020) Identification of polyphenolic compounds and hepatoprotective activity of artichoke (Cynara scolymus L.) edible part extracts in rats. Egypt J Chem 63(6):2273–2285
Kamel HN, Slattery M (2005) Terpenoids of sinularia.: chemistry and biomedical applications. Pharm Biol 43:253–269
Wu Y-J, Neoh C-A, Tsao C-Y, Su J-H, Li H-H (2015) Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci 16:16469–16482
Cheng T-C, Din Z-H, Su J-H, Wu Y-J, Liu C-I (2017) Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and urokinase through the PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar Drugs 15:238
Shanmugam MK, Shen H, Tang FR, Arfuso F, Rajesh M, Wang L, Kumar AP, Bian J, Goh BC, Bishayee A, Sethi G (2018) Potential role of genipin in cancer therapy. Pharmacol Res 133:195–200
Wang N, Zhu M, Tsao S-W, Man K, Zhang Z, Feng Y (2012) Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma. PLoS ONE 7:e46318–e46318
Shindo S, Hosokawa Y, Hosokawa I, Ozaki K, Matsuo T (2014) Genipin inhibits MMP-1 and MMP-3 release from TNF-a-stimulated human periodontal ligament cells. Biochimie 107:391–395
García-Vilas JA, Martínez-Poveda B, Quesada AR, Medina MÁ (2015) Aeroplysinin-1, a sponge-derived multi-targeted bioactive marine drug. Mar Drugs 14:1–1
Martínez-Poveda B, García-Vilas JA, Cárdenas C, Melgarejo E, Quesada AR, Medina MA (2013) The brominated compound aeroplysinin-1 inhibits proliferation and the expression of key pro- inflammatory molecules in human endothelial and monocyte cells. PLoS ONE 8:e55203–e55203
Ciccone L, Vandooren J, Nencetti S, Orlandini E (2021) Natural marine and terrestrial compounds as modulators of matrix metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s disease. Pharmaceuticals 14:86
Di Bari G, Gentile E, Latronico T, Corriero G, Fasano A, Nonnis Marzano C, Liuzzi GM (2015) Inhibitory effect of aqueous extracts from marine sponges on the activity and expression of gelatinases A (MMP-2) and B (MMP-9) in rat astrocyte cultures. PLoS ONE 10:e0129322–e0129322
Negri A, Naponelli V, Rizzi F, Bettuzzi S (2018) molecular targets of epigallocatechin-gallate (EGCG): a special focus on signal transduction and cancer. Nutrients 10:1936
Sazuka M, Imazawa H, Shoji Y, Mita T, Hara Y, Isemura M (1997) Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci Biotechnol Biochem 61:1504–1506
Chowdhury A, Nandy SK, Sarkar J, Chakraborti T, Chakraborti S (2017) Inhibition of pro-/active MMP-2 by green tea catechins and prediction of their interaction by molecular docking studies. Mol Cell Biochem 427:111–122
Sarkar J, Nandy SK, Chowdhury A, Chakraborti T, Chakraborti S (2016) Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed Pharmacother 84:340–347
Desai K, Sivakami S (2004) Spirulina: the wonder food of the 21st Century. Asia-Pacific Biotech News 8:1298–1302
Miranda M, Cintra R, Barros SBDM, Mancini-Filho J (1998) Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res 31:1075–1079
Abdel-Daim MM, Farouk SM, Madkour FF, Azab SS (2015) Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol Immunotoxicol 37:126–139
Pérez-Juárez A, Chamorro G, Alva-Sánchez C, Paniagua-Castro N, Pacheco-Rosado J (2016) Neuroprotective effect of Arthrospira (Spirulina) platensis against kainic acid-neuronal death. Pharm Biol 54:1408–1412
Salama AF, Abdel-Hamid NM, El-Sheekh M, Tosson E, Gabr AM (2017) Spirulina platensis microalgae protects against diethyl nitrosamine carcinogenic effect on female albino rats. Alex J Vet Sci 53:167–179
Samuels R, Mani U, Iyer U, Nayak U (2002) Hypocholesterolemic effect of Spirulina in patients with hyperlipidemic nephrotic syndrome. J Med Food 5:91–96
Chen Y-H, Chang G-K, Kuo S-M, Huang S-Y, Hu I-C, Lo Y-L, Shih S-R (2016) Well-tolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality. Sci Rep 6:24253
Kepekçi RA, Polat S, Çelik A, Bayat N, Saygideger SD (2013) Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem 141:1972–1979
Kunte M, Desai K (2017) The inhibitory effect of C-phycocyanin containing protein extract (C-PC Extract) on human matrix metalloproteinases (MMP-2 and MMP-9) in hepatocellular cancer cell line (HepG2). Protein J 36:186–195
Chaudhary AK, Singh M, Bharti AC, Asotra K, Sundaram S, Mehrotra R (2010) Genetic polymorphisms of matrix metalloproteinases and their inhibitors in potentially malignant and malignant lesions of the head and neck. J Biomed Sci 17:10–10
Kousidou OC, Mitropoulou T, Roussidis A, Kletsas D, Theocharis A, Karamanos N (2005) Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int J Oncol 26:1101–1109
Ramkita N, Falamy R, Farishal A (2021) Potential of genistein isoflavones as supportive therapy in prostate cancer. Cancer 2:63–70
Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M (2016) Anticancer efficacy of polyphenols and their combinations. Nutrients 8:552
Yan W, Zhang W, Sun L, Liu Y, You G, Wang Y, Kang C, You Y, Jiang T (2011) Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res 1411:108–115
Wang H, Qi C, Wan D (2021) MicroRNA-377–3p targeting MMP-16 inhibits ovarian cancer cell growth, invasion, and interstitial transition. Ann Transl Med 9:124
Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E, Wang H, Chen Y, Liu K, Shao Z, Shang Z (2018) Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res 37:1–15
Abba M, Patil N, Allgayer H (2014) MicroRNAs in the regulation of MMPs and metastasis. Cancers (Basel) 6:625–645
Li L, Li H (2013) Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol Ther 14:796–805
Xu B, Li Y-Y, Ma J, Pei F-X (2016) Roles of microRNA and signaling pathway in osteoarthritis pathogenesis. J Zhejiang Univ 17:200–208
Ruan H, Liang X, Zhao W, Ma L, Zhao Y (2017) The effects of microRNA-183 promots cell proliferation and invasion by targeting MMP-9 in endometrial cancer. Biomed Pharmacother 89:812–818
Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang L, Zhang H, Chen X, Yang Y, Liu G (2013) miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin β1 and matrix metalloproteinase2 (MMP2). PLoS ONE 8:e70192
Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M (2016) Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget 7:72758–72766
Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S, Ge R, Jiang S, Li G, Chen Y, He M-L, Kung H-F, Lai L, Lin MC (2009) microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res 1269:158–165
Wu H, Liu L, Zhu JM (2019) MiR-93-5p inhibited proliferation and metastasis of glioma cells by targeting MMP2. Eur Rev Med Pharmacol Sci 23:9517–9524
Cheng ZH, Luo C, Guo ZL (2019) MicroRNA-130b-5p accelerates the migration and invasion of osteosarcoma via binding to TIMP2. Eur Rev Med Pharmacol Sci 23:9267–9276
Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM (2008) MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol 28:5369–5380
Costa PM, Cardoso AL, Custódia C, Cunha P, Pereira de Almeida L, Pedroso de Lima MC (2015) MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: a new multimodal gene therapy approach for glioblastoma. J Control Release 207:31–39
Hwang SJ, Seol HJ, Park YM, Kim KH, Gorospe M, Nam D-H, Kim HH (2012) MicroRNA-146a suppresses metastatic activity in brain metastasis. Mol Cells 34:329–334
Hu Y, Ou Y, Wu K, Chen Y, Sun W (2012) miR-143 inhibits the metastasis of pancreatic cancer and an associated signaling pathway. Tumour Biol 33:1863–1870
Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T, Ito H, Oshimura M, Ochiya T (2011) MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther 19:1123–1130
Wang Q, Cai J, Wang J, Xiong C, Zhao J (2014) MiR-143 inhibits EGFR-signaling-dependent osteosarcoma invasion. Tumour Biol 35:12743–12748
Simonova OA, Kuznetsova EB, Tanas AS, Rudenko VV, Poddubskaya EV, Kekeeva TV, Trotsenko ID, Larin SS, Kutsev SI, Zaletaev DV (2020) Abnormal hypermethylation of CpG dinucleotides in promoter regions of matrix metalloproteinases genes in breast cancer and its relation to epigenomic subtypes and HER2 overexpression. Biomedicines 8:116
Falzone L, Salemi R, Travali S, Scalisi A, McCubrey JA, Candido S, Libra M (2016) MMP-9 overexpression is associated with intragenic hypermethylation of MMP9 gene in melanoma. Aging (Albany NY) 8:933
Klassen LM, Chequin A, Manica GC, Biembengut IV, Toledo MB, Baura VA, Pedrosa FDO, Ramos EA, Costa FF, De Souza EM (2018) MMP9 gene expression regulation by intragenic epigenetic modifications in breast cancer. Gene 642:461–466
Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A, van Gool R, Sexton DJ, Kuang G, Rank D, Hogan S, Pazmany C, Ma YL, Schoonbroodt S, Nixon AE, Ladner RC, Hoet R, Henderikx P, TenHoor C, Rabbani SA, Valentino ML, Wood CR, Dransfield DT (2009) Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res 69:1517–1526
Lemaître V, D’Armiento J (2006) Matrix metalloproteinases in development and disease. Birth defects research. Part C. Embryo Today 78:1–10
Paemen L, Martens E, Masure S, Opdenakker G (1995) Monoclonal antibodies specific for natural human neutrophil gelatinase B used for affinity purification, quantitation by two-site ELISA and inhibition of enzymatic activity. Eur J Biochem 234:759–765
Martens E, Leyssen A, Van Aelst I, Fiten P, Piccard H, Hu J, Descamps FJ, Van den Steen PE, Proost P, Van Damme J, Liuzzi GM, Riccio P, Polverini E, Opdenakker G (2007) A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochem Biophys Acta 1770:178–186
Hu J, Van den Steen PE, Houde M, Ilenchuk TT, Opdenakker G (2004) Inhibitors of gelatinase B/matrix metalloproteinase-9 activity comparison of a peptidomimetic and polyhistidine with single-chain derivatives of a neutralizing monoclonal antibody. Biochem Pharmacol 67:1001–1009
Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, Lee M, O’Sullivan C, Barry-Hamilton V, Ghermazien H, Mikels-Vigdal A, Garcia CA, Jorgensen B, Velayo AC, Wang R, Adamkewicz JI, Smith V (2015) Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PLoS ONE 10:e0127063
Sela-Passwell N, Kikkeri R, Dym O, Rozenberg H, Margalit R, Arad-Yellin R, Eisenstein M, Brenner O, Shoham T, Danon T, Shanzer A, Sagi I (2011) Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential. Nat Med 18:143–147
Abdel-Hamid NM, Abass SA, Mohamed AA, Muneam Hamid D (2018) Herbal management of hepatocellular carcinoma through cutting the pathways of the common risk factors. Biomed Pharmacother 107:1246–2125
Mitropoulou TN, Tzanakakis GN, Kletsas D, Kalofonos HP, Karamanos NK (2003) Letrozole as a potent inhibitor of cell proliferation and expression of metalloproteinases (MMP-2 and MMP-9) by human epithelial breast cancer cells. Int J Cancer 104:155–160
Falardeau P, Champagne P, Poyet P, Hariton C, Dupont É (2001) Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Seminars in oncology. Elsevier, Amsterdam, pp 620–625
Author information
Authors and Affiliations
Contributions
SA: Collection of data and manuscript drafting. NM: Suggestion of the article outline and title, revision of the article, preparation to publication. Both authors equally contributed to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
KFS University Committee of Scientific Research approved the work.
Consent for publication
Accept.
Consent for participation
NOt applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Hamid, N.M., Abass, S.A. Matrix metalloproteinase contribution in management of cancer proliferation, metastasis and drug targeting. Mol Biol Rep 48, 6525–6538 (2021). https://doi.org/10.1007/s11033-021-06635-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06635-z