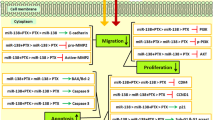
Abstract
Combination therapy has been considered as a potential method to overcome the BC chemoresistance. MicroRNAs (miRs) have been suggested as a therapeutic factor in the combination therapy of BC. This project aimed at examining the possible activity and molecular function of miR-424-5p and Taxol combination in the human BC cell line. MDA-MB-231 cells were treated with miR-424-5p mimics and Taxol, in a combined manner or separately. We used the MTT test for assessing the cell proliferation. In addition, flow-cytometry was used for evaluating apoptosis and cell-cycle. Expression levels of underlying molecular factors of miR-424-5p were assessed using western-blotting and qRT-PCR. The obtained results demonstrated that miR-424-5p repressed BC cell proliferation and sensitized these cells to Taxol treatment through the induction of apoptosis. Further investigations showed that miR-424-5p might increase BC chemosensitivity through the regulation of apoptosis-related factors including P53, Caspase-3, Bcl-2, and Bax as well as the proliferation-related gene c-Myc. Moreover, miR-424-5p restoration in combination with Taxol treatment decreased the colony formation by regulating Oct-4 and led to G2 arrest via modulating Cdk-2 expression. Western-blotting demonstrated that miR-424-5p may perform its anti-chemoresistance role by regulating the PD-L1 expression and controlling PTEN/PI3K/AKT/mTOR. Overall, the upregulation of miR-424-5p was indicated to upregulate the sensitivity of BC cells to treatment with Taxol. MiR-424-5p might regulate the chemosensitivity of the BC cell line by modulating PD-L1 and controlling the PTEN/mTOR axis. Therefore, the combination of miR-424-5p with Taxol would represent a novel procedure to treat against BC.
Graphic abstract






Similar content being viewed by others
Data availability
They would be available if requested by editor and reviewers.
References
Dastmalchi N, Safaralizadeh R, Baradaran B, Hosseinpourfeizi M, Baghbanzadeh A (2020) An update review of deregulated tumor suppressive microRNAs and their contribution in various molecular subtypes of breast cancer. Gene 729:144301
Momenimovahed Z, Salehiniya H (2019) Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 11:151–164
Maeda H, Khatami M (2018) Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med 7:11
Nedeljkovic M, Damjanovic A (2019) Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells 8(9):957
Abu Samaan TM, Samec M, Liskova A, Kubatka P, Busselberg D (2019) Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules 9(12):789
Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM et al (2007) Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf 6:609–621
Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S et al (2017) Combination therapy in combating cancer. Oncotarget 8:38022–38043
Ghasabi M, Majidi J, Mansoori B, Mohammadi A, Shomali N et al (2019) The effect of combined miR-200c replacement and cisplatin on apoptosis induction and inhibition of gastric cancer cell line migration. J Cell Physiol 234:22581–22592
Byler S, Goldgar S, Heerboth S, Leary M, Housman G et al (2014) Genetic and epigenetic aspects of breast cancer progression and therapy. Anticancer Res 34:1071–1077
Loh HY, Norman BP, Lai KS, Rahman N, Alitheen NBM et al (2019) The regulatory role of microRNAs in breast cancer. Int J Mol Sci 20(19):4940
Dastmalchi N, Safaralizadeh R, Banan Khojasteh SM (2019) The correlation between microRNAs and helicobacter pylori in gastric cancer. Pathog Dis. https://doi.org/10.1093/femspd/ftz039
Mansoori B, Mohammadi A, Ghasabi M, Shirjang S, Dehghan R et al (2019) miR-142-3p as tumor suppressor miRNA in the regulation of tumorigenicity, invasion and migration of human breast cancer by targeting Bach-1 expression. J Cell Physiol 234:9816–9825
Chakraborty C, Sharma AR, Sharma G, Sarkar BK, Lee SS (2018) The novel strategies for next-generation cancer treatment: miRNA combined with chemotherapeutic agents for the treatment of cancer. Oncotarget 9:10164–10174
Baldassari F, Zerbinati C, Galasso M, Corra F, Minotti L et al (2018) Screen for microRNA and drug interactions in breast cancer cell lines points to miR-126 as a modulator of CDK4/6 and PIK3CA inhibitors. Front Genet 9:174
Wang J, Wang S, Zhou J, Qian Q (2018) miR-424-5p regulates cell proliferation, migration and invasion by targeting doublecortin-like kinase 1 in basal-like breast cancer. Biomed Pharmacother 102:147–152
Dastmalchi N, Hosseinpourfeizi MA, Khojasteh SMB, Baradaran B, Safaralizadeh R (2020) Tumor suppressive activity of miR-424-5p in breast cancer cells through targeting PD-L1 and modulating PTEN/PI3K/AKT/mTOR signaling pathway. Life Sci 259:118239
Xu S, Tao Z, Hai B, Liang H, Shi Y et al (2016) miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat Commun 7:11406
Rodriguez-Barrueco R, Nekritz EA, Bertucci F, Yu J, Sanchez-Garcia F et al (2017) miR-424(322)/503 is a breast cancer tumor suppressor whose loss promotes resistance to chemotherapy. Genes Dev 31:553–566
Wu X, Li Y, Liu X, Chen C, Harrington SM et al (2019) Corrigendum to “Targeting B7–H1 (PD-L1) sensitizes cancer cells to chemotherapy” [Heliyon 4 (12) (December 2018) e01039]. Heliyon 5:e01309
Choi HJ, Heo JH, Park JY, Jeong JY, Cho HJ et al (2019) A novel PI3K/mTOR dual inhibitor, CMG002, overcomes the chemoresistance in ovarian cancer. Gynecol Oncol 153:135–148
Miao Y, Zheng W, Li N, Su Z, Zhao L et al (2017) MicroRNA-130b targets PTEN to mediate drug resistance and proliferation of breast cancer cells via the PI3K/Akt signaling pathway. Sci Rep 7:41942
Valovka T, Schonfeld M, Raffeiner P, Breuker K, Dunzendorfer-Matt T et al (2013) Transcriptional control of DNA replication licensing by Myc. Sci Rep 3:3444
Higuchi Y (2003) Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem Pharmacol 66:1527–1535
Du Z, Jia D, Liu S, Wang F, Li G et al (2009) Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia 57:724–733
Yang J, Nie J, Ma X, Wei Y, Peng Y et al (2019) Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer 18:26
Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274:113–126
Oualla K, El-Zawahry HM, Arun B, Reuben JM, Woodward WA et al (2017) Novel therapeutic strategies in the treatment of triple-negative breast cancer. Ther Adv Med Oncol 9:493–511
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121
Jeong YJ, Kang JS, Lee SI, So DM, Yun J et al (2016) Breast cancer cells evade paclitaxel-induced cell death by developing resistance to dasatinib. Oncol Lett 12:2153–2158
Gupta N, Gupta P, Srivastava SK (2019) Penfluridol overcomes paclitaxel resistance in metastatic breast cancer. Sci Rep 9:5066
Walker FE (1993) Paclitaxel (TAXOL): side effects and patient education issues. Semin Oncol Nurs 9:6–10
Si W, Shen J, Zheng H, Fan W (2019) The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics 11:25
Du H, Xu Q, Xiao S, Wu Z, Gong J et al (2019) MicroRNA-424-5p acts as a potential biomarker and inhibits proliferation and invasion in hepatocellular carcinoma by targeting TRIM29. Life Sci 224:1–11
Fang Y, Liang X, Xu J, Cai X (2018) miR-424 targets AKT3 and PSAT1 and has a tumor-suppressive role in human colorectal cancer. Cancer Manag Res 10:6537–6547
Lu L, Wu M, Lu Y, Zhao Z, Liu T et al (2019) MicroRNA-424 regulates cisplatin resistance of gastric cancer by targeting SMURF1 based on GEO database and primary validation in human gastric cancer tissues. Onco Targets Ther 12:7623–7636
Zhang HL, Wang P, Lu MZ, Zhang SD, Zheng L (2019) c-Myc maintains the self-renewal and chemoresistance properties of colon cancer stem cells. Oncol Lett 17:4487–4493
Wu Y, Chen W, Xu ZP, Gu W (2019) PD-l1 distribution and perspective for cancer immunotherapy-blockade, knockdown, or inhibition. Front Immunol 10:2022
Deng J, Bai X, Feng X, Ni J, Beretov J et al (2019) Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer 19:618
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y et al (2007) Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA 104:16158–16163
Martin A, Odajima J, Hunt SL, Dubus P, Ortega S et al (2005) Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1). Cancer Cell 7:591–598
Opyrchal M, Salisbury JL, Iankov I, Goetz MP, McCubrey J et al (2014) Inhibition of Cdk2 kinase activity selectively targets the CD44(+)/CD24(-)/Low stem-like subpopulation and restores chemosensitivity of SUM149PT triple-negative breast cancer cells. Int J Oncol 45:1193–1199
Liu TM, Wu YN, Guo XM, Hui JH, Lee EH et al (2009) Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev 18:1013–1022
Almozyan S, Colak D, Mansour F, Alaiya A, Al-Harazi O et al (2017) PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int J Cancer 141:1402–1412
Lu CS, Shieh GS, Wang CT, Su BH, Su YC et al (2017) Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer. Oncotarget 8:30844–30858
Mohiuddin IS, Wei SJ, Kang MH (2020) Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim Biophys Acta Mol Basis Dis 1866:165432
Wang G, Zhou H, Gu Z, Gao Q, Shen G (2018) Oct4 promotes cancer cell proliferation and migration and leads to poor prognosis associated with the survivin/STAT3 pathway in hepatocellular carcinoma. Oncol Rep 40:979–987
Tsai LL, Yu CC, Chang YC, Yu CH, Chou MY (2011) Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med 40:621–628
Yao X, Tu Y, Xu Y, Guo Y, Yao F et al (2020) Endoplasmic reticulum stress confers 5-fluorouracil resistance in breast cancer cell via the GRP78/OCT4/lncRNA MIAT/AKT pathway. Am J Cancer Res 10:838–855
Acknowledgements
This study was considered as a part of the project entitled “The evaluation of non coding RNAs role in human cancers” which is conducted at University of Tabriz. We would like to thank Tabriz Immunology Research Center, Iran for providing facilities to carry out this project.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Ethical approval
This project does not contain any investigations with human clinical samples or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dastmalchi, N., Safaralizadeh, R., Hosseinpourfeizi, M.A. et al. MicroRNA-424-5p enhances chemosensitivity of breast cancer cells to Taxol and regulates cell cycle, apoptosis, and proliferation. Mol Biol Rep 48, 1345–1357 (2021). https://doi.org/10.1007/s11033-021-06193-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06193-4