Abstract
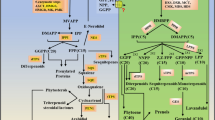
Increasing lipid content using metabolic engineering methods in different parts of plant, including, leaves and stem can be considered as an innovative platform for achieving more energy and biofuel in more green habits. Two key enzymes, including, diacylglycerol acyltransferase (DGAT) and phospholipid:diacylglycerol acyltransferase (PDAT) catalyze the final step of TAG assembly. WRINKLED1 (WRI1) is one of the important transcription factors which regulate the fatty acid biosynthesis network and TAG accumulation by balancing carbon flux between carbohydrates and lipids. In addition, oleosin encoding gene (OLE) can protect TAGs from degradation by packing into oil bodies. In the current study, four important genes involved in TAG assembly and protection (i.e., AtDGAT1 and AtPDAT, AtWRI1, and AtOle) were overexpressed under a constitutive promoter in rice crop. TAG content of transgenic seeds increased significantly (P ≤ 0.05) by 26% in compared with those of control plants. Oleic and palmitic acid contents were significantly increased by 28% (from 32 to 41) and 27% (11 to 14) in seeds of transgenic plants in compared with controls, respectively. Our results showed an increase in the total grain and leaf oil contents by 70% (from 1.1 to 1.87%) and 22.5% (from 1.88 to 2.3%) in the metabolically engineered lines, respectively. This is the first report of transformation in rice for enhancing oil content and energy density in its seeds and vegetative parts. Such metabolically engineered crops would be cultivated for production much more oils in seeds and straw for food and biodiesel consequently.





Similar content being viewed by others
References
Maravi DK, Kumar S, Sharma PK et al (2016) Ectopic expression of AtDGAT1, encoding diacylglycerol O-acyltransferase exclusively committed to TAG biosynthesis, enhances oil accumulation in seeds and leaves of Jatropha. Biotechnol Biofuels 9:1–13. https://doi.org/10.1186/s13068-016-0642-7
Reynolds KB, Taylor MC, Zhou XR et al (2015) Metabolic engineering of medium-chain fatty acid biosynthesis in Nicotiana benthamiana plant leaf lipids. Front Plant Sci 6:1–14. https://doi.org/10.3389/fpls.2015.00164
Yang Y, Benning C (2018) Functions of triacylglycerols during plant development and stress. Curr Opin Biotechnol 49:191–198. https://doi.org/10.1016/j.copbio.2017.09.003
Alameldin H, Izadi-Darbandi A, Smith SA et al (2017) Metabolic engineering to increase the corn seed storage lipid quantity and change its compositional quality. Crop Sci 57, 1854–1864. https://doi.org/10.2135/cropsci2016.06.0513
Alameldin H, Izadi-Darbandi A, Smith SA et al (2017) Production of seed-like storage lipids and increase in oil bodies in corn (Maize; Zea mays L) vegetative biomass. Ind Crops Prod 108:526–534. https://doi.org/10.1016/j.indcrop.2017.07.021
Liu F, Xia Y, Wu L et al (2015) Enhanced seed oil content by overexpressing genes related to triacylglyceride synthesis. Gene 557:163–171. https://doi.org/10.1016/j.gene.2014.12.029
Zale J, Jung JH, Kim JY et al (2016) Metabolic engineering of sugarcane to accumulate energy-dense triacylglycerols in vegetative biomass. Plant Biotechnol J 14:661–669. https://doi.org/10.1111/pbi.12411
Vanhercke T, El Tahchy A, Liu Q et al (2014) Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol J 12:231–239. https://doi.org/10.1111/pbi.12131
Bhunia RK, Kaur R, Maiti MK (2016) Metabolic engineering of fatty acid biosynthetic pathway in sesame (Sesamum indicum L): assembling tools to develop nutritionally desirable sesame seed oil. Phytochem Rev 15:799–811. https://doi.org/10.1007/s11101-015-9424-2
Wayne LL, Gachotte DJ, Walsh TA (2019) Transgenic and genome editing approaches for modifying plant oils. Transgenic Plants. Humana Press, New York, pp 367–394. https://doi.org/10.1007/978-1-4939-8778-8_23
Zhang Q, Yu R, Sun D et al (2017) PrLPAAT4, a putative lysophosphatidic acid acyltransferase from Paeonia rockii, plays an important role in seed fatty. Acid Biosynth Mol 22:2–14. https://doi.org/10.3390/molecules22101694
Kim H, Park JH, Kim AY et al (2016) Functional analysis of diacylglycerol acyltransferase1 genes from Camelina sativa and effects of CsDGAT1B overexpression on seed mass and storage oil content in C. sativa. Plant Biotechnol Rep 10:141–153. https://doi.org/10.1007/s11816-016-0394-7
Marmon S, Sturtevant D, Herrfurth C et al (2017) Two acyltransferases contribute differently to linolenic acid levels in seed oil. Plant Physiol 173:2081–2095. https://doi.org/10.1104/pp.16.01865
Xu C, Shanklin J (2016) Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu Rev Plant Biol 67:179–206. https://doi.org/10.1146/annurev-arplant-043015-111641
Rosli R, Chan PL, Chan KL et al (2018) In silico characterization and expression profiling of the diacylglycerol acyltransferase gene family (DGAT1, DGAT2, DGAT3 and WS/DGAT) from oil palm, Elaeis guineensis. Plant Sci 275:84–96. https://doi.org/10.1016/j.plantsci.2018.07.011
Savadi S, Naresh V, Kumar V, Bhat SR (2015) Seed-specific overexpression of Arabidopsis DGAT1 in Indian mustard (Brassica juncea) increases seed oil content and seed weight. Botany 94:177–184. https://doi.org/10.1139/cjb-2015-0218
Turchetto-Zolet AC, Christoff AP, Kulcheski FR et al (2016) Diversity and evolution of plant diacylglycerol acyltransferase (DGATs) unveiled by phylogenetic, gene structure and expression analyses. Genet Mol Biol 39:524–538. https://doi.org/10.1590/1678-4685-GMB-2016-0024
Li R, Yu K, Hildebrand DF (2010) DGAT1, DGAT2 and PDAT expression in seeds and other tissues of epoxy and hydroxy fatty acid accumulating plants. Lipids 45:145–157. https://doi.org/10.1007/s11745-010-3385-4
Ye J, Qu J, Chua NH (2018) Overexpression of a transcription factor increases lipid content in a woody perennial Jatropha curcas. Front Plant Sci 9:1–13. https://doi.org/10.3389/fpls.2018.01479
Ruuska SA, Girke T, Benning C, Ohlrogge JB (2002) Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14: 1191–1206. https://doi.org/10.1105/tpc.114.134296
Grimberg Å, Carlsson AS, Marttila S et al (2015) Transcriptional transitions in Nicotiana benthamiana leaves upon induction of oil synthesis by WRINKLED1 homologs from diverse species and tissues. BMC Plant Biol 15(1):1–117. https://doi.org/10.1186/s12870-015-0579-1
Jin J, Sun Y, Qu J et al (2017) Transcriptome and functional analysis reveals hybrid vigor for oil biosynthesis in oil palm. Sci Rep 7(439):1–12. https://doi.org/10.1038/s41598-017-00438-8
Baud S, Mendoza MS, To A et al (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50:825–838. https://doi.org/10.1111/j.1365-313X.2007.03092.x
Zulu NN, Popko J, Zienkiewicz K et al (2017) Heterologous co-expression of a yeast diacylglycerol acyltransferase (ScDGA1) and a plant oleosin (AtOLEO3) as an efficient tool for enhancing triacylglycerol accumulation in the marine diatom Phaeodactylum tricornutum. Biotechnol Biofuels 10(187):1–14. https://doi.org/10.1186/s13068-017-0874-1
Chen K, YinY, Li S et al (2019) Genome-wide identification and functional analysis of oleosin genes in Brassica napus L. BMC Plant Biol 19(1):294. https://doi.org/10.1186/s12870-019-1891-y
Ding M, Lou H, Chen W et al (2020) Comparative transcriptome analysis of the genes involved in lipid biosynthesis pathway and regulation of oil body formation in Torreya grandis kernels. Ind Crops Prod 145:112051. https://doi.org/10.1016/j.indcrop.2019.112051
Vega R, Vásquez N, Espinoza AM et al (2009) Histology of somatic embryogenesis in rice (Oryza sativa cv 5272). Rev Biol Trop 57:141–150. https://doi.org/10.15517/rbt.v57i0.21291
Ma W, Kong Q, Arondel V et al (2013) Wrinkled1, a ubiquitous regulator in oil accumulating tissues from Arabidopsis embryos to oil palm mesocarp. PLoS ONE 8(7):1–13. https://doi.org/10.1371/journal.pone.0068887
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Juliano BO, Perez CM, Blakeney AB et al (1981) International cooperative testing on the amylose content of milled rice. Starch (Stärke) 33:157–162. https://doi.org/10.1002/star.19810330504
Xia J, Igor VS, Beomsoo H, David SW (2015) MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 43(W1):W251–W257. https://doi.org/10.1093/nar/gkv380
Lim JS, Manan ZA, Alwi SR, Hashim H (2012) A review on utilisation of biomass from rice industry as a source of renewable energy. Renew Sustain Energy Rev 16:3084–3094. https://doi.org/10.1016/j.rser.2012.02.051
Rogalski M, Carrer H (2011) Engineering plastid fatty acid biosynthesis to improve food quality and biofuel production in higher plants. Plant Biotechnol J 9:554–564. https://doi.org/10.1111/j.1467-7652.2011.00621.x
Acknowledgements
We sincerely thank Magyar Krueger of Michigan State University Biomass Conversion Research Laboratory for assisting with the oil extraction for GC–MS fatty acid profiling. We also thank Professor Emeritus John Ohlrogge of Michigan State University for the availability of the pWRI1 construct, and Dr. Changcheng Xu of the Brookhaven National Laboratory for the availability of pOle.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Ali Izadi-Darbandi], [Mehdi Younessi-Hamzekhanlu] and [Mariam Sticklen]. The first draft of the manuscript was written by [Ali Izadi-Darbandi and Mehdi Younessi-Hamzekhanlu] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there are no conflict of interest to disclose.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izadi-Darbandi, A., Younessi-Hamzekhanlu, M. & Sticklen, M. Metabolically engineered rice biomass and grain using genes associated with lipid pathway show high level of oil content. Mol Biol Rep 47, 7917–7927 (2020). https://doi.org/10.1007/s11033-020-05837-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05837-1