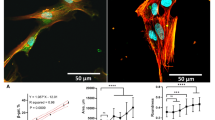
Abstract
Here, we document changes in cell motility and organization of the contractile apparatus of human umbilical cord Wharton's jelly mesenchymal stem cells (MSCWJ-1) in the process of replicative senescence. Colocalization dynamics of F-actin and actin-binding proteins (myosin-9, α-actinin-4, RhoA) were examined in the MSCWJ-1 cell line. The results show that nuclear-cytoplasmic redistribution of RhoA occurs during replicative senescence, with maximal RhoA/nucleus colocalization evident at passage 15. At that time point, decreases in colocalization, namely myosin-9/F-actin and α-actinin-4/F-actin, were seen and myosin-9 was found in cytosolic extracts in the assembly-incompetent form. Using an automated intravital confocal cytometry system and quantitative analysis of MSCWJ-1 movements, we found that changes in cytoskeletal organization correlate with cell motility characteristics over a time period from passages 9 to 38. The factors examined (cytoskeleton structure, cell motility) indicate that the process by which cells transition to replicative senescence is best represented as three stages. The first stage lasts from cell culture isolation to passage 15 and is characterized by: accumulation of actin-binding proteins in assembly-incompetent forms; nuclear RhoA accumulation; and an increase in movement tortuosity. The second stage extends from passages 15 to 28 and is characterized by: an increase in the structural integrity of the actin cytoskeleton; exit of RhoA and alpha-actinin-4 from the nucleus; and a decrease in path tortuosity. The third stage extends from passage 28 to 38 and is marked by: a plateau in actin cytoskeleton structural integrity; significant decreases in nuclear RhoA levels; and decreases in cell speed.






Similar content being viewed by others
References
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 25(11):2896–2902. https://doi.org/10.1634/stemcells.2007-0637
Carvalho MM, Teixeira FG, Reis RL, Sousa N, Salgado AJ (2011) Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Curr Stem Cell Res Ther 6(3):221–228. https://doi.org/10.2174/157488811796575332
Guiducci S, Manetti M, Romano E et al (2011) Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheumat Dis. https://doi.org/10.1136/ard.2011.150607
Huang YC, Leung VY, Lu WW, Luk KD (2013) The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J 13(3):352–362. https://doi.org/10.1016/j.spinee.2012.12.005
Luo J, Zhao X, Tan Z, Su Z, Meng F, Zhang M (2013) Mesenchymal-like progenitors derived from human embryonic stem cells promote recovery from acute kidney injury via paracrine actions. Cytotherapy 15(6):649–662. https://doi.org/10.1016/j.jcyt.2013.01.009
Ando Y, Matsubara K, Ishikawa J, Fujio M, Shohara R, Hibi H, Ueda M, Yamamoto A (2014) Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 61:82–90. https://doi.org/10.1016/j.bone.2013.12.029
Hendijani F, Javanmard SH, Sadeghi-Aliabadi H (2015) Human Wharton's jelly mesenchymal stem cell secretome display antiproliferative effect on leukemia cell line and produce additive cytotoxic effect in combination with doxorubicin. Tissue Cell 47(3):229–234. https://doi.org/10.1016/j.tice.2015.01.005
Hendijani F, Javanmard SH, Rafiee L, Sadeghi-Aliabadi H (2015) Effect of human Wharton's jelly mesenchymal stem cell secretome on proliferation, apoptosis and drug resistance of lung cancer cells. Res Pharm Sci 10(2):134
Danieli P, Malpasso G, Ciuffreda MC, Gnecchi M (2016) Testing the paracrine properties of human mesenchymal stem cells using conditioned medium. Mesenchymal stem cells. Humana Press, New York, pp 445–456
Julianto I, Rindastuti Y (2016) Topical delivery of mesenchymal stem cells" secretomes" in wound repair. Acta Med Indonesiana 48(3):217–220
Vulcano F, Milazzo L, Ciccarelli C, Eramo A, Sette G, Mauro A, Macioce G, Martinelli A, La Torre R, Casalbore P, Hassan HJ, Giampaolo A (2016) Wharton's jelly mesenchymal stromal cells have contrasting effects on proliferation and phenotype of cancer stem cells from different subtypes of lung cancer. Exp Cell Res 345(2):190–198. https://doi.org/10.1016/j.yexcr.2016.06.003
Zachar L, Bačenková D, Rosocha J (2016) Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflam Res 9:231. https://doi.org/10.2147/JIR.S121994
Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD (2008) Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE 3(5):e2213. https://doi.org/10.1371/journal.pone.0002213
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24(22):2463–2479. https://doi.org/10.1101/gad.1971610
Redaelli S, Bentivegna A, Foudah D, Miloso M, Redondo J, Riva G, Baronchelli S, Dalprà L, Tredici G (2012) From cytogenomic to epigenomic profiles: monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells. Stem Cell Res Ther 3(6):47. https://doi.org/10.1186/scrt138
Estrada JC, Torres Y, Benguria A, Dopazo A, Roche E, Carrera-Quintanar L, Pérez RA, Enríquez JA, Torres R, Ramírez JC, Samper E, Bernad A (2013) Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis 4(6):e691. https://doi.org/10.1038/cddis.2013.211
Savickienė J, Baronaitė S, Zentelytė A, Treigytė G, Navakauskienė R (2016) Senescence-associated molecular and epigenetic alterations in mesenchymal stem cell cultures from amniotic fluid of normal and fetus-affected pregnancy. Stem Cells Int 2016:2019498. https://doi.org/10.1155/2016/2019498
Danisovic L, Oravcova L, Krajciova L, Varchulova Novakova Z, Bohac M, Varga I, Vojtassak J (2017) Effect of long-term culture on the biological and morphological characteristics of human adipose tissue-derived stem cells. J Physiol Pharmacol 68(1):149–158
Koltsova AM, Krylova TA, Musorina AS, Zenin VV, Turilova VI, Yakovleva TK, Poljanskaya GG (2018) The dynamics of cell properties during long-term cultivation of two lines of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord. Cell Tissue Biol 12(1):7–19. https://doi.org/10.1134/S1990519X1801011X
Alessio N, Pipino C, Mandatori D, Di Tomo P, Ferone A, Marchiso M, Melone MAB, Peluso G, Pandolfi A, Galderisi U (2018) Mesenchymal stromal cells from amniotic fluid are less prone to senescence compared to those obtained from bone marrow: an in vitro study. J Cell Physiol 233(11):8996–9006. https://doi.org/10.1002/jcp.26845
Niedernhofer LJ, Gurkar AU, Wang Y, Vijg J, Hoeijmakers JH, Robbins PD (2018) Nuclear genomic instability and aging. Annu Rev Biochem 87:295–322. https://doi.org/10.1146/annurev-biochem-062917-012239
Yu J, Shi J, Zhang Y, Zhang Y, Huang Y, Chen Z, Yang J (2018) The replicative senescent mesenchymal stem/stromal cells defect in DNA damage response and anti-oxidative capacity. Int J Med Sci 15(8):771. https://doi.org/10.7150/ijms.24635
Geißler S, Textor M, Kühnisch J, Könnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN (2012) Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS ONE 7(12):e52700. https://doi.org/10.1371/journal.pone.0052700
Bertolo A, Gemperli A, Gruber M, Gantenbein B, Baur M, Pötzel T, Stoyanov J (2015) In vitro cell motility as a potential mesenchymal stem cell marker for multipotency. Stem Cells Transl Med 4(1):84–90. https://doi.org/10.5966/sctm.2014-0156
Turinetto V, Vitale E, Giachino C (2016) Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci 17(7):1164. https://doi.org/10.3390/ijms17071164
Zhang T, Wang P, Liu Y, Zhou J, Shi Z, Cheng K, Huang T, Wang X, Yang GL, Yang B, Ma S, Guan F (2018) Overexpression of FOXQ1 enhances anti-senescence and migration effects of human umbilical cord mesenchymal stem cells in vitro and in vivo. Cell Tissue Res 373(2):379–393. https://doi.org/10.1007/s00441-018-2815-0
Vasiliev JM (1991) Polarization of pseudopodial activities: cytoskeletal mechanisms. J Cell Sci 98(1):1–4
Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM (2002) Mechanisms of polarization of the shape of fibroblasts and epitheliocytes: Separation of the roles of microtubules and Rho-dependent actin–myosin contractility. Proc Natl Acad Sci USA 99(16):10452–10457. https://doi.org/10.1073/pnas.152339899
Larsen M, Tremblay ML, Yamada KM (2003) Phosphatases in cell–matrix adhesion and migration. Nat Rev Mol Cell Biol 4(9):700. https://doi.org/10.1038/nrm1199
Le Clainche C, Carlier MF (2008) Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev 88(2):489–513. https://doi.org/10.1152/physrev.00021.2007
Wang D, Jang DJ (2009) Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Can Res 69(20):8200–8207. https://doi.org/10.1158/0008-5472.CAN-09-1976
Özcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, Galderisi U (2016) Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 8(7):1316
Moujaber O, Fishbein F, Omran N, Liang Y, Colmegna I, Presley JF, Stochaj U (2019) Cellular senescence is associated with reorganization of the microtubule cytoskeleton. Cell Mol Life Sci 76(6):1169–1183. https://doi.org/10.1007/s00018-018-2999-1
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Dj P, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. https://doi.org/10.1080/14653240600855905
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) Image J2: ImageJ for the next generation of scientific image data. BMC Bioinform 18(1):529. https://doi.org/10.1186/s12859-017-1934-z
Adler J, Pagakis SN, Parmryd I (2008) Replicate-based noise corrected correlation for accurate measurements of colocalization. J Microsc 230(1):121–133. https://doi.org/10.1111/j.1365-2818.2008.01967.x
Bergholm F, Adler J, Parmryd I (2010) Analysis of bias in the apparent correlation coefficient between image pairs corrupted by severe noise. J Math Imaging Vis 37(3):204–219. https://doi.org/10.1007/s10851-010-0200-z
Mukaka MM (2012) A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24(3):69–71
Sakashita H, Ohashi K, Ozawa K, Tsubouchi Y (2015) The CQ1 confocal quantitative image cytometer and its application to biological measurement. Yokogawa Tech Rep Engl Ed 58(1):29–33
McLean DJ, Skowron Volponi MA (2018) trajr: An R package for characterisation of animal trajectories. Ethology 124(6):440–448. https://doi.org/10.1111/eth.12739
Bovet P, Benhamou S (1988) Spatial analysis of animals' movements using a correlated random walk model. J Theor Biol 131(4):419–433. https://doi.org/10.1016/S0022-5193(88)80038-9
Cheung A, Zhang S, Stricker C, Srinivasan MV (2007) Animal navigation: the difficulty of moving in a straight line. Biol Cybern 97(1):47–61. https://doi.org/10.1007/s00422-007-0158-0
Benhamou S (2004) How to reliably estimate the tortuosity of an animal's path: straightness, sinuosity, or fractal dimension? J Theor Biol 229(2):209–220. https://doi.org/10.1016/j.jtbi.2004.03.016
Bobkov DE, Aizenshtadt AA, Kropacheva IV, Pinaev GP (2012) Isolation of tropomyosin particles from cultured cell cytosol and their protein composition assay. Cell Tissue Biol 6(2):137–146. https://doi.org/10.1134/S1990519X12020046
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680. https://doi.org/10.1038/227680a0
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76(9):4350–4354. https://doi.org/10.1073/pnas.76.9.4350
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org Accessed 30 Nov 2019
Wilson EB (1927) Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 22(158):209–212. https://doi.org/10.2307/2276774
Husson F, Lê S, Pagès J (2017) Exploratory multivariate analysis by example using R. Chapman and Hall, New York
Dobson AJ, Barnett AG (2008) An introduction to generalized linear models. Chapman and Hall, New York
Lawley DN, Maxwell AE (1971) Factor analysis as statistical method. Butterworths, London
Terpilowski M (2019) scikit-posthocs: pairwise multiple comparison tests in Python. J Open Source Softw 4:1169
Shapiro SS, Francia RS (1972) An approximate analysis of variance test for normality. J Am Stat Assoc 67(337):215–216. https://doi.org/10.1080/01621459.1972.10481232
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621. https://doi.org/10.2307/2280779
Wilcoxon F (1992) Individual comparisons by ranking methods. Breakthroughs in statistics. Springer, New York, pp 196–202
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25(3):585–621. https://doi.org/10.1016/0014-4827(61)90192-6
Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL (2003) Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302(5651):1775–1779. https://doi.org/10.1126/science.1090772
Shutova MS, Asokan SB, Talwar S, Assoian RK, Bear JE, Svitkina TM (2017) Self-sorting of nonmuscle myosins IIA and IIB polarizes the cytoskeleton and modulates cell motility. J Cell Biol 216(9):2877–2889. https://doi.org/10.1083/jcb.201705167
Shutova MS, Svitkina TM (2018) Mammalian nonmuscle myosin II comes in three flavors. Biochem Biophys Res Commun 506(2):394–402. https://doi.org/10.1016/j.bbrc.2018.03.103
Elliott H, Fischer RS, Myers KA, Desai RA, Gao L, Chen CS, Adelstein RS, Waterman CM, Danuser G (2015) Myosin II controls cellular branching morphogenesis and migration in three dimensions by minimizing cell-surface curvature. Nat Cell Biol 17(2):137–147. https://doi.org/10.1038/ncb3092
Grenklo S, Hillberg L, Zhao Rathje LS, Pinaev G, Schutt CE, Lindberg U (2008) Tropomyosin assembly intermediates in the control of microfilament system turnover. Eur J Cell Biol 87(11):905–920. https://doi.org/10.1016/j.ejcb.2008.06.006
Bobkov DE, Kropacheva IV (2017) The effect of lysophosphatidic acid on the composition of cytoplasmic protein complexes that contain myosin-9 and tropomyosin. Cell Tissue Biol 11(3):197–204. https://doi.org/10.1134/S1990519X17030026
Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10(11):778. https://doi.org/10.1038/nrm2786
Hofmann WA, Arduini A, Nicol SM, Camacho CJ, Lessard JL, Fuller-Pace FV, de Lanerolle P (2009) SUMOylation of nuclear actin. J Cell Biol 186(2):193–200. https://doi.org/10.1083/jcb.200905016
Alonso A, Greenlee M, Matts J, Kline J, Davis KJ, Miller RK (2015) Emerging roles of sumoylation in the regulation of actin, microtubules, intermediate filaments, and septins. Cytoskeleton 72(7):305–339. https://doi.org/10.1002/cm.21226
Salah H, Li M, Cacciani N, Gastaldello S, Ogilvie H, Akkad H, Namuduri AV, Morbidoni V, Artemenko KA, Balogh G, Martinez-Redondo V, Jannig P, Hedström Y, Dworkin B, Bergquist J, Ruas J, Vigh L, Salviati L, Larsson L (2016) The chaperone co-inducer BGP-15 alleviates ventilation-induced diaphragm dysfunction. Sci Transl Med 8(350):350ra103. https://doi.org/10.1126/scitranslmed.aaf7099
Senger F, Pitaval A, Ennomani H, Kurzawa L, Blanchoin L, Théry M (2019) Spatial integration of mechanical forces by alpha-actinin establishes actin network symmetry. J Cell Sci 132(22):236604. https://doi.org/10.1242/jcs.236604
Hu S, Grobe H, Guo Z, Wang YH, Doss BL, Pan M, Ladoux B, Bershadsky AD, Zaidel-Bar R (2019) Reciprocal regulation of actomyosin organization and contractility in non-muscle cells by tropomyosins and alpha-actinins. Mol Biol Cell 30(16):2025–2036. https://doi.org/10.1091/mbc.E19-02-0082
Bolshakova A, Petukhova O, Turoverova L, Tentler D, Babakov V, Magnusson KE, Pinaev G (2007) Extra-cellular matrix proteins induce re-distribution of α-actinin-1 and α-actinin-4 in A431 cells. Cell Biol Int 31(4):360–365. https://doi.org/10.1016/j.cellbi.2007.01.021
Babakov VN, Petukhova OA, Turoverova LV, Kropacheva IV, Tentler DG, Bolshakova AV, Podolskaya EP, Magnusson KE, Pinaev GP (2008) RelA/NF-κB transcription factor associates with α-actinin-4. Exp Cell Res 314(5):1030–1038. https://doi.org/10.1016/j.yexcr.2007.12.001
Khotin MG, Turoverova LV, Podolskaya EP, Krasnov IA, Solovyeva AV, Aksenova VY, Magnusson KE, Pinaev GP, Tentler DG (2009) Analysis of nuclear protein complexes comprising α-actinin-4 by 2D-electrophoresis and mass spectrometry. Cell Tissue Biol 3(5):431. https://doi.org/10.1134/S1990519X09050058
Lomert E, Turoverova L, Kriger D, Aksenov ND, Nikotina AD, Petukhov A, Mittenberg AG, Panyushev NV, Khotin M, Volkov K, Barlev NA, Tentler D (2018) Co-expression of RelA/p65 and ACTN4 induces apoptosis in non-small lung carcinoma cells. Cell Cycle 17(5):616–626. https://doi.org/10.1080/15384101.2017.1417709
Barbolina MV, Adley BP, Kelly DL, Fought AJ, Scholtens DM, Shea LD, Stack MS (2008) Motility-related actinin alpha-4 is associated with advanced and metastatic ovarian carcinoma. Lab Invest 88(6):602. https://doi.org/10.1038/labinvest.2008.25
Hsu KS, Kao HY (2013) Alpha-actinin 4 and tumorigenesis of breast cancer. Vitam Horm 93:323–351. https://doi.org/10.1016/B978-0-12-416673-8.00005-8
An HT, Yoo S, Ko J (2016) α-Actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of Snail expression and β-catenin stabilization in cervical cancer. Oncogene 35(45):5893. https://doi.org/10.1038/onc.2016.117
Huveneers S, Danen EH (2009) Adhesion signaling–crosstalk between integrins, Src and Rho. J Cell Sci 122(8):1059–1069. https://doi.org/10.1242/jcs.039446
Ridley AJ, Hall A (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70(3):389–399. https://doi.org/10.1016/0092-8674(92)90163-7
Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116(2):167–179. https://doi.org/10.1016/s0092-8674(04)00003-0
Guilluy C, Dubash AD, García-Mata R (2011) Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus. Nat Protoc 6(12):2050. https://doi.org/10.1038/nprot.2011.411
Kim JG, Islam R, Cho JY, Jeong H, Cap KC, Park Y, Hossain AJ, Park JB (2018) Regulation of RhoA GTPase and various transcription factors in the RhoA pathway. J Cell Physiol 233(9):6381–6392. https://doi.org/10.1002/jcp.26487
Tiurin-Kuz'min PA, Vorotnikov AV, Tkachuk VA (2013) Molecular mechanisms of gradient sensing in mesenchymal cells. Ross Fiziol Zhurnal Imeni Sechenova 99(3):294–312
Li L, Nørrelykke SF, Cox EC (2008) Persistent cell motion in the absence of external signals: a search strategy for eukaryotic cells. PLoS ONE 3(5):e2093. https://doi.org/10.1371/journal.pone.0002093
Khaitlina SY (2001) Functional specificity of actin isoforms. Int Rev Cytol 202:35–98. https://doi.org/10.1016/s0074-7696(01)02003-4
Dubash AD, Guilluy C, Srougi MC, Boulter E, Burridge K, García-Mata R (2011) The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PLoS ONE 6(2):e17380. https://doi.org/10.1371/journal.pone.0017380
Karperien A (2013) FracLac for ImageJ. Charles Sturt University, Colombo
Qian AR, Li D, Han J, Gao X, Di SM, Zhang W, Shang P (2012) Fractal dimension as a measure of altered actin cytoskeleton in MC3T3-E1 cells under simulated microgravity using 3-D/2-D clinostats. IEEE Trans Biomed Eng 59(5):1374–1380. https://doi.org/10.1109/TBME.2012.2187785
Alhussein G, Shanti A, Farhat IAH, Timraz SBH, Alwahab NSA, Pearson YE, Martin MN, Christoforou N, Teo JCM (2016) A spatiotemporal characterization method for the dynamic cytoskeleton. Cytoskeleton 73(5):221–232. https://doi.org/10.1002/cm.21297
Waliszewski P (2016) The quantitative criteria based on the fractal dimensions, entropy, and lacunarity for the spatial distribution of cancer cell nuclei enable identification of low or high aggressive prostate carcinomas. Front Physiol 7:34. https://doi.org/10.3389/fphys.2016.00034
Acknowledgements
The authors are deeply grateful to their colleagues: Dr. G. Stein and M. Vorobyov, for their expert assistance in confocal microscopy; S. Boykov, for assistance in computational trajectory analysis; I. Kropacheva and Dr. A. Koltsova, for technical help and general support; and E. Ramsay, for his assistance with writing and editing.
Funding
This work was carried out as part of a State Assignment of the Institute of Cytology RAS (No. AAAA-A19-119020190093–9).
Author information
Authors and Affiliations
Contributions
Danila Bobkov and Galina Poljanskaya designed the research and analyzed data. Anastasia Polyanskaya performed immunofluorescence and western blot work. Anastasia Musorina maintained the cell line and performed β-galactosidase analysis. Ekaterina Lomert carried out intravital confocal cytometry. Sergey Shabelnikov performed chromatographic separations. The first draft of the manuscript was written by Danila Bobkov, and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (AVI 131657 kb)
Rights and permissions
About this article
Cite this article
Bobkov, D., Polyanskaya, A., Musorina, A. et al. Replicative senescence in MSCWJ-1 human umbilical cord mesenchymal stem cells is marked by characteristic changes in motility, cytoskeletal organization, and RhoA localization. Mol Biol Rep 47, 3867–3883 (2020). https://doi.org/10.1007/s11033-020-05476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05476-6