Abstract
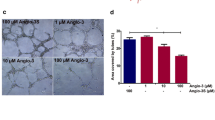
Tumor angiogenesis allows tumor cells to grow and migrate toward the bloodstream and initiate metastasis. The interactions of vascular endothelial growth factors (VEGF) A and B, as the important regulating factors for blood vessel growth, with VEGFR1 and VEGFR2 trigger angiogenesis process. Thus, preventing these interactions led to the effective blockade of VEGF/VEGFRs signaling pathways. In this study, the inhibitory effect of a 23-mer linear peptide (VGB4), which binds to both VEGFR1 and VEGFR2, on VEGF-stimulated Human Umbilical Vein Endothelial Cells (HUVECs) and highly metastatic human breast cancer cell MDA-MB-231 proliferation was examined using MTT assay. To assess the anti-migratory potential of VGB4, HUVECs and also MDA-MB-231 cells wound healing assay was carried out at 48 and 72 h. In addition, downstream signaling pathways of VEGF associated with cell migration and invasion were investigated by quantification of mRNA and protein expression using real-time quantitative PCR and western blot in 4T1 tumor tissues and MDA-MB-231 cells. The results revealed that VGB4 significantly impeded proliferation of HUVECs and MDA-MB-231 cells, in a dose- and time-dependent manner, and migration of HUVECs and MDA-MB-231 cells for a prolonged time. We also observed statistically significant reduction of the transcripts and protein levels of focal adhesion kinase (FAK), Paxillin, matrix metalloproteinase-2 (MMP-2), RAS-related C3 botulinum substrate 1 (Rac1), P21-activated kinase-2 (PAK-2) and Cofilin-1 in VGB4-treated 4T1 tumor tissues compared to controls. The protein levels of phospho-VEGFR1, phospho-VEGFR2, Vimentin, β-catenin and Snail were markedly decreased in both VGB4-treated MDA-MB-231 cells and VGB4-treated 4T1 tumor tissues compared to controls as evidenced by western blotting. These results, in addition to our previous studies, confirm that dual blockage of VEGFR1 and VEGFR2, due to the inactivation of diverse signaling mediators, effectively suppresses tumor growth and metastasis.




Similar content being viewed by others
References
Bielenberg DR, Zetter BR (2015) The contribution of angiogenesis to the process of metastasis. Cancer J 21(4):267. https://doi.org/10.1097/PPO.0000000000000138
Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84(3):359–369. https://doi.org/10.1016/S0092-8674(00)81280-5
Ivaska J, Heino J (2010) Interplay between cell adhesion and growth factor receptors: from the plasma membrane to the endosomes. Cell Tissue Res 339(1):111. https://doi.org/10.1007/s00441-009-0857-z
Avraham HK, Lee T-H, Koh Y, Kim T-A, Jiang S, Sussman M, Samarel AM, Avraham S (2003) Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J Biol Chem 278(38):36661–36668. https://doi.org/10.1074/jbc.M301253200
Abedi H, Zachary I (1997) Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem 272(24):15442–15451. https://doi.org/10.1074/jbc.272.24.15442
Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6(1):56. https://doi.org/10.1038/nrm1549
Golubovskaya V, Beviglia L, Xu L-H, Earp HS, Craven R, Cance W (2002) Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem 277(41):38978–38987. https://doi.org/10.1074/jbc.M205002200
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2(5):249. https://doi.org/10.1038/35010517
Mon NN, Ito S, Senga T, Hamaguchi M (2006) FAK signaling in neoplastic disorders: a linkage between inflammation and cancer. Ann N Y Acad Sci 1086(1):199–212. https://doi.org/10.1196/annals.1377.019
Hu Y-L, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC, Chien S (2014) FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep 4:6024. https://doi.org/10.1038/srep06024
Brown MC, Turner CE (2004) Paxillin: adapting to change. Physiol Rev 84(4):1315–1339. https://doi.org/10.1152/physrev.00002.2004
Kumar R, Gururaj AE, Barnes CJ (2006) p21-activated kinases in cancer. Nat Rev Cancer 6(6):459. https://doi.org/10.1038/nrc1892
Renkema GH, Pulkkinen K, Saksela K (2002) Cdc42/Rac1-mediated activation primes PAK2 for superactivation by tyrosine phosphorylation. Mol Cell Biol 22(19):6719–6725. https://doi.org/10.1128/MCB.22.19.6719-6725.2002
Mira J-P, Benard V, Groffen J, Sanders LC, Knaus UG (2000) Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci 97(1):185–189. https://doi.org/10.1073/pnas.97.1.185
Holm C, Rayala S, Jirström K, Stål O, Kumar R, Landberg G (2006) Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst 98(10):671–680. https://doi.org/10.1093/jnci/djj185
Arias-Romero LE, Chernoff J (2010) p21-activated kinases in Erbb2-positive breast cancer: A new therapeutic target? Small GTPases 1(2):5839–5849. https://doi.org/10.4161/sgtp.1.2.14109
Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S (1999) Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285(5429):895–898. https://doi.org/10.1126/science.285.5429.895
Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, Bokoch GM (2007) Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell 13(5):646–662. https://doi.org/10.1016/j.devcel.2007.08.011
Rider L, Oladimeji P, Diakonova M (2013) PAK1 regulates breast cancer cell invasion through secretion of matrix metalloproteinases in response to prolactin and three-dimensional collagen IV. Mol Endocrinol 27(7):1048–1064. https://doi.org/10.1210/me.2012-1322
Poola I, DeWitty RL, Marshalleck JJ, Bhatnagar R, Abraham J, Leffall LD (2005) Identification of MMP-1 as a putative breast cancer predictive marker by global gene expression analysis. Nat Med 11(5):481. https://doi.org/10.1038/nm1243
Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3(12):a005058. https://doi.org/10.1101/cshperspect.a005058
Smith BN, Burton LJ, Henderson V, Randle DD, Morton DJ, Smith BA, Taliaferro-Smith L, Nagappan P, Yates C, Zayzafoon M (2014) Snail promotes epithelial mesenchymal transition in breast cancer cells in part via activation of nuclear ERK2. PLoS ONE 9(8):e104987. https://doi.org/10.1371/journal.pone.0104987
Liu C-Y, Lin H-H, Tang M-J, Wang Y-K (2015) Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 6(18):15966
Behelgardi MF, Zahri S, Mashayekhi F, Mansouri K, Asghari SM (2018) A peptide mimicking the binding sites of VEGF-A and VEGF-B inhibits VEGFR-1/-2 driven angiogenesis, tumor growth and metastasis. Sci Rep 8(1):17924. https://doi.org/10.1038/s41598-018-36394-0
Bauer AT, Bürgers HF, Rabie T, Marti HH (2010) Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cerebral Blood Flow Metab 30(4):837–848. https://doi.org/10.1038/jcbfm.2009.248
Lee JH, Shim JW, Choi YJ, Heo K, Yang K (2013) The combination of sorafenib and radiation preferentially inhibits breast cancer stem cells by suppressing HIF-1α expression. Oncol Rep 29(3):917–924. https://doi.org/10.3892/or.2013.2228
Goel HL, Mercurio AM (2013) VEGF targets the tumour cell. Nat Rev Cancer 13(12):871. https://doi.org/10.1038/nrc3627
Yan J-D, Liu Y, Zhang Z-Y, Liu G-Y, Xu J-H, Liu L-Y, Hu Y-M (2015) Expression and prognostic significance of VEGFR-2 in breast cancer. Pathol Res Pract 211(7):539–543. https://doi.org/10.1016/j.prp.2015.04.003
Wang Y, Shi J, Chai K, Ying X, Zhou P (2013) The role of Snail in EMT and tumorigenesis. Curr Cancer Drug Targets 13(9):963–972
Luo M, Hou L, Li J, Shao S, Huang S, Meng D, Liu L, Feng L, Xia P, Qin T (2016) VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-κB and β-catenin. Cancer Lett 373(1):1–11. https://doi.org/10.1016/j.canlet.2016.01.010
Ning Q, Liu C, Hou L, Meng M, Zhang X, Luo M, Shao S, Zuo X, Zhao X (2013) Vascular endothelial growth factor receptor-1 activation promotes migration and invasion of breast cancer cells through epithelial-mesenchymal transition. PLoS ONE 8(6):e65217. https://doi.org/10.1371/journal.pone.0065217
Gille J, Heidenreich R, Pinter A, Schmitz J, Boehme B, Hicklin DJ, Henschler R, Breier G (2007) Simultaneous blockade of VEGFR-1 and VEGFR-2 activation is necessary to efficiently inhibit experimental melanoma growth and metastasis formation. Int J Cancer 120(9):1899–1908. https://doi.org/10.1002/ijc.22531
Sadremomtaz A, Mansouri K, Alemzadeh G, Safa M, Rastaghi AE, Asghari SM (2018) Dual blockade of VEGFR1 and VEGFR2 by a novel peptide abrogates VEGF-driven angiogenesis, tumor growth, and metastasis through PI3K/AKT and MAPK/ERK1/2 pathway. Biochimica et Biophysica Acta (BBA) 1862(12):2688–2700. https://doi.org/10.1016/j.bbagen.2018.08.013
Sadremomtaz A, Kobarfard F, Mansouri K, Mirzanejad L, Asghari SM (2018) Suppression of migratory and metastatic pathways via blocking VEGFR1 and VEGFR2. J Recept Signal Trans 38(5–6):432–441. https://doi.org/10.1080/10799893.2019.1567785
Assareh E, Mehrnejad F, Mansouri K, Rastaghi ARE, Naderi-Manesh H, Asghari SM (2019) A cyclic peptide reproducing the α1 helix of VEGF-B binds to VEGFR-1 and VEGFR-2 and inhibits angiogenesis and tumor growth. Biochem J 476(4):645–663. https://doi.org/10.1042/BCJ20180823
Shiojima I, Walsh K (2002) Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 90(12):1243–1250. https://doi.org/10.1161/01.RES.0000022200.71892.9F
Wang F, Yamauchi M, Muramatsu M, Osawa T, Tsuchida R, Shibuya M (2011) RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3K/Akt pathway. J Biol Chem 286(11):9097–9106. https://doi.org/10.1074/jbc.M110.165605
Butti R, Das S, Gunasekaran VP, Yadav AS, Kumar D, Kundu GC (2018) Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer 17(1):34. https://doi.org/10.1186/s12943-018-0797-x
Wei Z, Shan Z, Shaikh ZA (2018) Epithelial-mesenchymal transition in breast epithelial cells treated with cadmium and the role of Snail. Toxicol Appl Pharmacol 344:46–55. https://doi.org/10.1016/j.taap.2018.02.022
Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M (2012) Turnover of focal adhesions and cancer cell migration. Int J Cell Biol. https://doi.org/10.1155/2012/310616
Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD, Hendrix MJ (2004) Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol 165(4):1087–1095. https://doi.org/10.1016/S0002-9440(10)63370-6
Megison ML, Stewart JE, Nabers HC, Gillory LA, Beierle EA (2013) FAK inhibition decreases cell invasion, migration and metastasis in MYCN amplified neuroblastoma. Clin Exp Metas 30(5):555–568. https://doi.org/10.1007/s10585-012-9560-7
Wendt MK, Schiemann WP (2009) Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-β signaling and metastasis. Breast Cancer Res 11(5):R68. https://doi.org/10.1186/bcr2360
Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J (2000) Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem 275(14):10661–10672. https://doi.org/10.1074/jbc.275.14.10661
Le Boeuf F, Houle F, Huot J (2004) Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J Biol Chem 279(37):39175–39185. https://doi.org/10.1074/jbc.M405493200
Lamalice L, Houle F, Huot J (2006) Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J Biol Chem 281(45):34009–34020. https://doi.org/10.1074/jbc.M603928200
Zhu X, Zhou W (2015) The emerging regulation of VEGFR-2 in triple-negative breast cancer. Front Endocrinol 6:159. https://doi.org/10.3389/fendo.2015.00159
Wang W, Eddy R, Condeelis J (2007) The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer 7(6):429. https://doi.org/10.1038/nrc2148
Sawano A, Takahashi T, Yamaguchi S, Shibuya M (1997) The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCγ. Biochem Biophys Res Commun 238(2):487–491. https://doi.org/10.1006/bbrc.1997.7327
Takahashi T, Yamaguchi S, Chida K, Shibuya M (2001) A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J 20(11):2768–2778. https://doi.org/10.1093/emboj/20.11.2768
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Farzaneh Behelgardi, M., Zahri, S., Gholami Shahvir, Z. et al. Targeting signaling pathways of VEGFR1 and VEGFR2 as a potential target in the treatment of breast cancer. Mol Biol Rep 47, 2061–2071 (2020). https://doi.org/10.1007/s11033-020-05306-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05306-9