Abstract
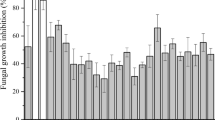
A recent spike in demand for chemical preservative free food has derived the scientific community to develop natural ways of food preservation. Therefore, bio-preservation could be considered as the great alternative over chemical ones owing to its potential to increase shelf-life and nutritional values of foodstuffs. In the present study, lactic acid producing bacterial species were isolated from rice rinsed water and identified by 16S rRNA gene sequencing as Lactobacillus plantarum BCH-1 (KX388380) and Lactobacillus coryniformis BCH-4 (KX388387). Antifungal metabolites from both Lactobacillus species were extracted by polarity-based solvents in which ethyl acetate showed remarkable antifungal activity against Aspergillus flavus and Aspergillus fumigatus by disc diffusion assay. Different organic acids and fatty acids have been identified by reversed-phase high-performance liquid chromatography (RP-HPLC) and gas chromatography–mass spectrometry (GC–MS) analysis, respectively. Lactic acid and citric acid were the major organic acids found in ethyl acetate fractions of L. plantarum and L. coryniformis, respectively. Similarly, 9,12-otadecadienoic acid (Z,Z)-methyl ester and hexadecanoic acid, methyl ester were the major fatty acids found in n-hexane fractions of L. plantarum and L. coryniformis respectively. Moreover, the isolation of novel antifungal metabolites from locally isolated Lactobacillus species was focused and it was revealed that organic acids are important contributors towards antifungal potential. A novel fatty acid (i.e. 12-hydroxydodecanoic acid) has also been explored and found as potential metabolite against filamentous fungi. Conclusively, various metabolites isolated from non-dairy source showed antifungal activity especially against Aspergillus species. Hence, these metabolites have been considered as a good choice for bio-preservation.








Similar content being viewed by others
References
Pitt JI (2000) Toxigenic fungi: which are important? Sabouraudia 38(Supplement_1):17–22. https://doi.org/10.1080/mmy.38.s1.17.22
Dalie D, Deschamps A, Richard-Forget F (2010) Lactic acid bacteria–potential for control of mould growth and mycotoxins: a review. Food Control 21(4):370–380. https://doi.org/10.1016/j.foodcont.2009.07.011
Pitt JI, Hocking AD (2009) Spoilage of stored, processed and preserved foods. In: Pitt JI, Hocking AD (eds) Fungi and food spoilage. Springer, Boston, pp 401–421
Vermeulen N, Gánzle MG, Vogel RF (2006) Influence of peptide supply and cosubstrates on phenylalanine metabolism of Lactobacillus sanfranciscensis dsm20451t and Lactobacillus plantarum tmw1.468. J Agric Food Chem 54(11):3832–3839. https://doi.org/10.1021/jf052733e
Filtenborg O, Frisvad JC, Thrane U (1996) Moulds in food spoilage. Int J Food Microbiol 33(1):85–102. https://doi.org/10.1016/0168-1605(96)01153-1
Nevarez L, Vasseur V, Debaets S, Barbier G (2010) Use of response surface methodology to optimise environmental stress conditions on Penicillium glabrum, a food spoilage mould. Fungal Biol 114(5–6):490–497. https://doi.org/10.1016/j.funbio.2010.03.011
Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J (2003) Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol Lett 219(1):129–135. https://doi.org/10.1016/S0378-1097(02)01207-7
Adebayo C, Aderiye B (2011) Suspected mode of antimycotic action of brevicin sg1 against Candida albicans and Penicillium citrinum. Food Control 22(12):1814–1820. https://doi.org/10.1016/j.foodcont.2011.04.013
Belguesmia Y, Rabesona H, Mounier J, Pawtowsky A, Le Blay G, Barbier G, Haertlé T, Chobert JM (2014) Characterization of antifungal organic acids produced by Lactobacillus harbinensis k.V9.31 np immobilized in gellan–xanthan beads during batch fermentation. Food Control 36(1):205–211. https://doi.org/10.1016/j.foodcont.2013.08.028
Black BA, Zannini E, Curtis JM, Gänzle MG (2013) Antifungal hydroxy-fatty acids produced during sourdough fermentation: microbial and enzymatic pathways, and antifungal activity in bread. Appl Environ Microbiol 79(6):1866–1873. https://doi.org/10.1128/AEM.03784-12
Gerez CL, Carbajo MS, Rollán G, Leal T, de FontValdez G (2010) Inhibition of citrus fungal pathogens by using lactic acid bacteria. J Food Sci 75(6):M354–M359. https://doi.org/10.1111/j.1750-3841.2010.01671.x
Kwak MK, Liu R, Kwon JO, Kim MK, Kim AH, Kang SO (2013) Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza a virus. J Microbiol 51(6):836–843. https://doi.org/10.1007/s12275-013-3521-y
Rouse S, Harnett D, Vaughan A, Sinderen Dv (2008) Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J Appl Microbiol 104(3):915–923. https://doi.org/10.1111/j.1365-2672.2007.03619.x
Schnürer J, Magnusson J (2005) Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Technol 16(1–3):70–78. https://doi.org/10.1016/j.tifs.2004.02.014
Ikeda DM, Weinert E, Chang KC, McGinn JM, Miller SA, Keliihoomalu C, DuPonte MW (2013) Natural farming: lactic acid bacteria. Sustain Agric 8:3–4
Nikita C, Hemangi D (2012) Isolation, identification and characterization of lactic acid bacteria from dairy sludge sample. J Environ Res Dev 7(1A):1–11
Lotte R, Durand M, Mbeutcha A, Ambrosetti D, Pulcini C, Degand N, Loeffler J, Ruimy R, Amiel J (2014) A rare case of histopathological bladder necrosis associated with Actinobaculum schaalii: the incremental value of an accurate microbiological diagnosis using 16S rDNA sequencing. Anaerobe 26:46–48. https://doi.org/10.1016/j.anaerobe.2014.01.005
Li R, Mock R, Huang Q, Abad J, Hartung J, Kinard G (2008) A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J Virol Methods 154(1–2):48–55. https://doi.org/10.1016/j.jviromet.2008.09.008
Martínez-Absalón S, Rojas-Solís D, Hernández-León R, Prieto-Barajas C, Orozco-Mosqueda MdC, Peña-Cabriales JJ, Sakuda S, Valencia-Cantero E, Santoyo G (2014) Potential use and mode of action of the new strain bacillus thuringiensis um96 for the biological control of the grey mould phytopathogen Botrytis cinerea. Biocontrol Sci Technol 24(12):1349–1362. https://doi.org/10.1080/09583157.2014.940846
Costa GN, Vilas-Bôas GT, Vilas-Boas LA, Miglioranza LH (2011) In silico phylogenetic analysis of lactic acid bacteria and new primer set for identification of Lactobacillus plantarum in food samples. Eur Food Res Technol 233(2):233–241. https://doi.org/10.1007/s00217-011-1508-7
Ström K, Sjögren J, Broberg A, Schnürer J (2002) Lactobacillus plantarum milab 393 produces the antifungal cyclic dipeptides cyclo (l-phe-l-pro) and cyclo (l-phe-trans-4-oh-l-pro) and 3-phenyllactic acid. Appl Environ Microbiol 68(9):4322–4327. https://doi.org/10.1128/AEM.68.9.4322-4327.2002
Wang H, Yan Y, Wang J, Zhang H, Qi W (2012) Production and characterization of antifungal compounds produced by Lactobacillus plantarum imau10014. PLoS ONE 7(1):e29452. https://doi.org/10.1371/journal.pone.0029452
Yang E, Chang H (2010) Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int J Food Microbiol 139(1–2):56–63. https://doi.org/10.1016/j.ijfoodmicro.2010.02.012
Bibi I, Nawaz H, Kamal S, Jilani K, Ruby T, Warsi F (2015) Investigation of catalytic properties of manganese peroxidase (mnp) produced from Agaricus bisporus A21 and its potential application in the biotransformation of xenobiotic compound. J Chem Soc Pak 37(5):859–868
Broberg A, Jacobsson K, Ström K, Schnürer J (2007) Metabolite profiles of lactic acid bacteria in grass silage. Appl Environ Microbiol 73(17):5547–5552. https://doi.org/10.1128/AEM.02939-06
Glöckner FO, Zaichikov E, Belkova N, Denissova L, Pernthaler J, Pernthaler A, Amann R (2000) Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of actinobacteria. Appl Environ Microbiol 66(11):5053–5065. https://doi.org/10.1128/AEM.66.11.5053-5065.2000
Tohno M, Kobayashi H, Nomura M, Kitahara M, Ohkuma M, Uegaki R, Cai Y (2012) Genotypic and phenotypic characterization of lactic acid bacteria isolated from Italian ryegrass silage. Anim Sci J 83(2):111–120. https://doi.org/10.1111/j.1740-0929.2011.00923.x
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H (2012) Introducing eztaxon-e: a prokaryotic 16 s rrna gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62(3):716–721. https://doi.org/10.1099/ijs.0.038075-0
Purkhold U, Pommerening-Röser A, Juretschko S, Schmid MC, Koops HP, Wagner M (2000) Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoa sequence analysis: implications for molecular diversity surveys. Appl Environ Microbiol 66(12):5368–5382. https://doi.org/10.1128/AEM.66.12.5368-5382.2000
Nuryana I, Andriani A, Lisdiyanti P (2019) Analysis of organic acids produced by lactic acid bacteria. IOP Conf Ser Earth Environ Sci 251(1):012054. https://doi.org/10.1088/1755-1315/251/1/012054
Dal Bello F, Clarke C, Ryan L, Ulmer H, Schober T, Ström K, Sjögren J, Van Sinderen D, Schnürer J, Arendt E (2007) Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain lactobacillus plantarum fst 1.7. J Cereal Sci 45(3):309–318. https://doi.org/10.1016/j.jcs.2006.09.004
Schillinger U, Villarreal JV (2010) Inhibition of Penicillium nordicum in MRS medium by lactic acid bacteria isolated from foods. Food Control 21(2):107–111. https://doi.org/10.1016/j.foodcont.2008.11.010
Reis FS, Stojković D, Soković M, Glamočlija J, Ćirić A, Barros L, Ferreira IC (2012) Chemical characterization of Agaricus bohusii, antioxidant potential and antifungal preserving properties when incorporated in cream cheese. Food Res Int 48(2):620–626. https://doi.org/10.1016/j.foodres.2012.06.013
Hunter DR, Segel IH (1973) Effect of weak acids on amino acid transport by Penicillium chrysogenum: evidence for a proton or charge gradient as the driving force. J Bacteriol 113(3):1184–1192
Niku-Paavola ML, Laitila A, Mattila‐Sandholm T, Haikara A (1999) New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol 86(1):29–35. https://doi.org/10.1046/j.1365-2672.1999.00632.x
Atanassova M, Choiset Y, Dalgalarrondo M, Chobert JM, Dousset X, Ivanova I, Haertlé T (2003) Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. Paracasei strain m3. Int J Food Microbiol 87(1–2):63–73. https://doi.org/10.1016/S0168-1605(03)00054-0
Axelsson L (1991) Lactobacillus reuteri, a member of the gut bacterial flora: Studies on antagonism, metabolism and genetics, 0531–0531
Piard J, Desmazeaud M (1991) Inhibiting factors produced by lactic acid bacteria. 1. Oxygen metabolites and catabolism end-products. Le lait 171(5):525–541. https://doi.org/10.1051/lait:1991541
Stratford M, Eklund T (2003) Organic acids and esters. Food Preserv. Springer, Boston, pp 48–84. https://doi.org/10.1007/978-0-387-30042-9_4
Freese E, Sheu CW, Galliers E (1973) Function of lipophilic acids as antimicrobial food additives. Nature 241(5388):321–325. https://doi.org/10.1038/241321a0
Hassan R, El-Kadi S, Sand M (2015) Effect of some organic acids on some fungal growth and their toxins production. Int J Adv Biol 2(1):1–11
Zalán Z, Hudáček J, Štětina J, Chumchalová J, Halász A (2010) Production of organic acids by lactobacillus strains in three different media. Eur Food Res Technol 230(3):395. https://doi.org/10.1007/s00217-009-1179-9
Axel C, Brosnan B, Zannini E, Peyer LC, Furey A, Coffey A, Arendt EK (2016) Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl Microbiol Biotechnol 100(4):1701–1711. https://doi.org/10.1007/s00253-015-7051-x
Di Biase M, Lavermicocca P, Lonigro SL, Valerio F (2014) Lactobacillus brevis-based bioingredient inhibits Aspergillus niger growth on pan bread. Ital J Agron 9:146–151
Ryu EH, Yang EJ, Woo ER, Chang HC (2014) Purification and characterization of antifungal compounds from Lactobacillus plantarum hd1 isolated from kimchi. Food Microbiol 41:19–26. https://doi.org/10.1016/j.fm.2014.01.011
Le Lay C, Coton E, Le Blay G, Chobert JM, Haertlé T, Choiset Y, Van Long NN, Meslet-Cladiere L, Mounier J (2016a) Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int J Food Microbiol 239:79–85. https://doi.org/10.1016/j.ijfoodmicro.2016.06.020
Le Lay C, Mounier J, Vasseur V, Weill A, Le Blay G, Barbier G, Coton E (2016) In vitro and in situ screening of lactic acid bacteria and propionibacteria antifungal activities against bakery product spoilage molds. Food Control 60:247–255. https://doi.org/10.1016/j.foodcont.2015.07.034
Anyasi TA, Jideani AI, Mchau GR (2015) Effect of organic acid pretreatment on some physical, functional and antioxidant properties of flour obtained from three unripe banana cultivars. Food Chem 172:515–522. https://doi.org/10.1016/j.foodchem.2014.09.120
Quitmann H, Fan R, Czermak P (2013) Acidic organic compounds in beverage, food, and feed production. Biotechnol Food Feed Addit. Springer, Berlin, pp 91–141
Sjögren J, Magnusson J, Broberg A, Schnürer J, Kenne L (2003) Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum milab 14. Appl Environ Microbiol 69(12):7554–7557. https://doi.org/10.1128/AEM.69.12.7554-7557.2003
Ndagano D, Lamoureux T, Dortu C, Vandermoten S, Thonart P (2011) Antifungal activity of 2 lactic acid bacteria of the Weissella genus isolated from food. J Food Sci 76(6):M305–M311. https://doi.org/10.1111/j.1750-3841.2011.02257.x
Acknowledgements
We are grateful to Higher Education Commission (HEC) Islamabad, Government of Pakistan for financial support. The work was supported by HEC [21-432/SRGP/H&D/2014].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bukhari, S.A., Salman, M., Numan, M. et al. Characterization of antifungal metabolites produced by Lactobacillus plantarum and Lactobacillus coryniformis isolated from rice rinsed water. Mol Biol Rep 47, 1871–1881 (2020). https://doi.org/10.1007/s11033-020-05281-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05281-1