Abstract
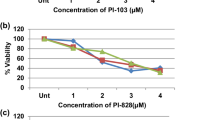
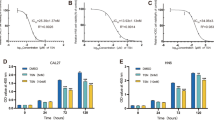
The purpose of this study was to evaluate the synergistic apoptotic effect of CKD-602 in combination with cisplatin on different p53 statuses of oral squamous cell carcinoma (OSCC) cell lines, YD-8, YD-9, and YD-38. MTT assays were used to evaluate the inhibitory effect of the treatments modality on cell growth. The combination index was calculated using CompuSyn software. Detection of cell death was carried out using the propidium iodide (PI)/RNase staining assay, Annexin V/PI double staining assay, and Western blotting. Combination treatment using CKD-602 and cisplatin inhibited proliferation and increased the apoptotic effect on the three OSCC cell lines. Apoptotic cell death was detected in the cell lines, and significant synergistic effects of CKD-602 in combination with cisplatin were observed despite the differences in p53 status. From these results, it was suggested that the combination of CKD-602 with cisplatin might be a potential therapeutic strategy for OSCC. In particular, cell line-specific therapy is necessary because of the differences in treatment response based on p53 status.




Similar content being viewed by others
Change history
24 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11033-021-06651-z
References
Korea Central Cancer Registry NCC (2016) Annual report of cancer statistics in Korea in 2014. Ministry of Health and Welfare
Dumache R (2017) Early diagnosis of oral squamous cell carcinoma by salivary microRNAs. Clin Lab 63(11):1771–1776. https://doi.org/10.7754/Clin.Lab.2017.170607
Li CC, Shen Z, Bavarian R, Yang F, Bhattacharya A (2018) Oral cancer: genetics and the role of precision medicine. Dent Clin N Am 62(1):29–46. https://doi.org/10.1016/j.cden.2017.08.002
Levi LE, Lalla RV (2018) Dental treatment planning for the patient with oral cancer. Dent Clin N Am 62(1):121–130. https://doi.org/10.1016/j.cden.2017.08.009
Villa A, Akintoye SO (2018) Dental management of patients who have undergone oral cancer therapy. Dent Clin N Am 62(1):131–142. https://doi.org/10.1016/j.cden.2017.08.010
Bundela S, Sharma A, Bisen PS (2015) Potential compounds for oral cancer treatment: resveratrol, nimbolide, lovastatin, bortezomib, vorinostat, berberine, pterostilbene, deguelin, andrographolide, and colchicine. PLoS ONE 10(11):e0141719. https://doi.org/10.1371/journal.pone.0141719
Jo DW, Kim YK, Yun PY (2016) The influence of p53 mutation status on the anti-cancer effect of cisplatin in oral squamous cell carcinoma cell lines. J Korean Assoc Oral Maxillofac Surg 42(6):337–344. https://doi.org/10.5125/jkaoms.2016.42.6.337
Cohen SM, Lippard SJ (2001) Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol 67:93–130
Qi X, Xu W, Xie J, Wang Y, Han S, Wei Z, Ni Y, Dong Y, Han W (2016) Metformin sensitizes the response of oral squamous cell carcinoma to cisplatin treatment through inhibition of NF-kappaB/HIF-1alpha signal axis. Sci Rep 6:35788. https://doi.org/10.1038/srep35788
Williams CJ, Whitehouse JM (1979) Cis-platinum: a new anticancer agent. Br Med J 1(6179):1689–1691
Von Hoff DD, Schilsky R, Reichert CM, Reddick RL, Rozencweig M, Young RC, Muggia FM (1979) Toxic effects of cis-dichlorodiammineplatinum (II) in man. Cancer Treat Rep 63(9–10):1527–1531
Tanaka T, Masuda H, Naito M, Tamai H (2001) Pretreatment with 5-fluorouracil enhances cytotoxicity and retention of DNA-bound platinum in a cisplatin resistant human ovarian cancer cell line. Anticancer Res 21(4a):2463–2469
Chen S, Hu H, Miao S, Zheng J, Xie Z, Zhao H (2017) Anti-tumor effect of cisplatin in human oral squamous cell carcinoma was enhanced by andrographolide via upregulation of phospho-p53 in vitro and in vivo. Tumour Biol 39(5):1010428317705330. https://doi.org/10.1177/1010428317705330
Crul M (2003) CKD-602. Chong Kun Dang. Curr Opin Investig Drugs (Lond, Engl: 2000) 4(12):1455–1459
Liu YQ, Li WQ, Morris-Natschke SL, Qian K, Yang L, Zhu GX, Wu XB, Chen AL, Zhang SY, Nan X, Lee KH (2015) Perspectives on biologically active camptothecin derivatives. Med Res Rev 35(4):753–789. https://doi.org/10.1002/med.21342
Ok YJ, Myoung H, Kim YK, Lee JH, Kim MJ, Yun PY (2009) Apoptotic effect of CKD-602 (Camtobell) on oral squamous cell carcinoma cell lines. Oral Oncol 45(3):266–272. https://doi.org/10.1016/j.oraloncology.2008.05.001
Kim YK, Koo NY, Yun PY (2015) Anticancer effects of CKD-602 (Camtobell (R)) via G2/M phase arrest in oral squamous cell carcinoma cell lines. Oncol Lett 9(1):136–142. https://doi.org/10.3892/ol.2014.2648
Lee JH, Lee JM, Kim JK, Ahn SK, Lee SJ, Kim MY, Jew SS, Park JG, Hong CI (1998) Antitumor activity of 7-[2-(N-isopropylamino)ethyl]-(20S)-camptothecin, CKD602, as a potent DNA topoisomerase I inhibitor. Arch Pharm Res 21(5):581–590
Lim S, Cho BC, Jung JY, Kim GM, Kim SH, Kim HR, Kim HS, Lim SM, Park JS, Lee JH, Kim D, Kim EY, Park MS, Kim YS, Kim SK, Chang J, Kim JH (2013) Phase II study of camtobell inj. (belotecan) in combination with cisplatin in patients with previously untreated, extensive stage small cell lung cancer. Lung Cancer (Amst Neth) 80(3):313–318. https://doi.org/10.1016/j.lungcan.2013.02.009
Hong J, Jung M, Kim YJ, Sym SJ, Kyung SY, Park J, Lee SP, Park JW, Cho EK, Jeong SH, Shin DB, Lee JH (2012) Phase II study of combined belotecan and cisplatin as first-line chemotherapy in patients with extensive disease of small cell lung cancer. Cancer Chemother Pharmacol 69(1):215–220. https://doi.org/10.1007/s00280-011-1689-6
Chan BA, Coward JI (2013) Chemotherapy advances in small-cell lung cancer. J Thorac Dis 5(Suppl 5):S565–S578. https://doi.org/10.3978/j.issn.2072-1439.2013.07.43
O’Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN, Friend S, Fornace AJ Jr, Kohn KW (1997) Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res 57(19):4285–4300
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31(15):1869–1883. https://doi.org/10.1038/onc.2011.384
Cutilli T, Leocata P, Dolo V, Altobelli E (2013) Evaluation of p53 protein as a prognostic factor for oral cancer surgery. Br J Oral Maxillofac Surg 51(8):922–927. https://doi.org/10.1016/j.bjoms.2013.05.150
Branch P, Masson M, Aquilina G, Bignami M, Karran P (2000) Spontaneous development of drug resistance: mismatch repair and p53 defects in resistance to cisplatin in human tumor cells. Oncogene 19(28):3138–3145. https://doi.org/10.1038/sj.onc.1203668
Bradford CR, Zhu S, Ogawa H, Ogawa T, Ubell M, Narayan A, Johnson G, Wolf GT, Fisher SG, Carey TE (2003) p53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck 25(8):654–661. https://doi.org/10.1002/hed.10274
Tao H, Zhu Y, Wang H, Lai B, Zhang C, Zhan X, Wang Y, Yang X, Yue W, Zhang H (2008) [Effects of p53 gene on drug resistance in human lung cancer cell lines]. Zhongguo fei ai za zhi = Chinese. J Lung Cancer 11(2):157–164. https://doi.org/10.3779/j.issn.1009-3419.2008.02.001
Dart DA, Picksley SM, Cooper PA, Double JA, Bibby MC (2004) The role of p53 in the chemotherapeutic responses to cisplatin, doxorubicin and 5-fluorouracil treatment. Int J Oncol 24(1):115–125
Andrews GA, Xi S, Pomerantz RG, Lin CJ, Gooding WE, Wentzel AL, Wu L, Sidransky D, Grandis JR (2004) Mutation of p53 in head and neck squamous cell carcinoma correlates with Bcl-2 expression and increased susceptibility to cisplatin-induced apoptosis. Head Neck 26(10):870–877. https://doi.org/10.1002/hed.20029
Bradford CR, Zhu S, Poore J, Fisher SG, Beals TF, Thoraval D, Hanash SM, Carey TE, Wolf GT (1997) p53 mutation as a prognostic marker in advanced laryngeal carcinoma. Department of veterans affairs laryngeal cancer cooperative study group. Arch Otolaryngol—Head Neck Surg 123(6):605–609
Lee EJ, Kim J, Lee SA, Kim EJ, Chun YC, Ryu MH, Yook JI (2005) Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp Mol Med 37(5):379–390. https://doi.org/10.1038/emm.2005.48
Piaskowski S, Zawlik I, Szybka M, Kulczycka-Wojdala D, Stoczynska-Fidelus E, Bienkowski M, Robak T, Kusinska R, Jesionek-Kupnicka D, Kordek R, Rieske P, Liberski PP (2010) Detection of p53 mutations in different cancer types is improved by cDNA sequencing. Oncol Lett 1(4):717–721. https://doi.org/10.3892/ol_00000125
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446. https://doi.org/10.1158/0008-5472.can-09-1947
Foucquier J, Guedj M (2015) Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 3(3):e00149. https://doi.org/10.1002/prp2.149
Wiman KG (2006) Strategies for therapeutic targeting of the p53 pathway in cancer. Cell Death Differ 13(6):921–926. https://doi.org/10.1038/sj.cdd.4401921
Chipuk JE, Green DR (2006) Dissecting p53-dependent apoptosis. Cell Death Differ 13(6):994–1002. https://doi.org/10.1038/sj.cdd.4401908
Miyashita T, Reed JC (1992) bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res 52(19):5407–5411
Kobayashi T, Ruan S, Clodi K, Kliche KO, Shiku H, Andreeff M, Zhang W (1998) Overexpression of Bax gene sensitizes K562 erythroleukemia cells to apoptosis induced by selective chemotherapeutic agents. Oncogene 16(12):1587–1591. https://doi.org/10.1038/sj.onc.1201681
Guo B, Cao S, Toth K, Azrak RG, Rustum YM (2000) Overexpression of Bax enhances antitumor activity of chemotherapeutic agents in human head and neck squamous cell carcinoma. Clin Cancer Res 6(2):718–724
Choi WS, Lee EH, Chung CW, Jung YK, Jin BK, Kim SU, Oh TH, Saido TC, Oh YJ (2001) Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2. J Neurochem 77(6):1531–1541
Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW (1998) Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17(9):1069–1078. https://doi.org/10.1038/sj.onc.1202034
Acknowledgements
This work was supported by the SNUBH Research Fund (Grant No. 02-2014-018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest related to this work.
Rights and permissions
About this article
Cite this article
Choi, H.S., Kim, YK. & Yun, PY. The role of p53 status on the synergistic effect of CKD-602 and cisplatin on oral squamous cell carcinoma cell lines. Mol Biol Rep 46, 617–625 (2019). https://doi.org/10.1007/s11033-018-4517-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4517-9